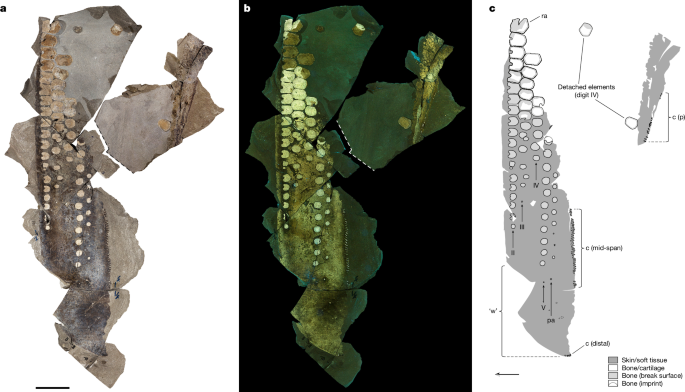
Fossil material
Initial efforts to re-assemble and mechanically prepare SSN8DOR11 were made at the time of its discovery in 2009. However, an investigative computed tomography (CT) scan in 2021 revealed the presence of additional bones that remained hidden within the matrix, and these were uncovered using a combination of chisels, dental picks and a microscribe.
An approximately 5-mm-thick section of a distal phalanx, an incipient ossification and three partial chondroderms were immersed in polyester resin (Araldite DBF, ABIC Kemi) to prevent shattering during slide preparation. Once embedded, approximately 1-mm-thick sections were cut from the blocks using a slow-speed diamond saw. Each section was attached to a petrographic slide with polyester resin and then ground to optical translucency. All sections were imaged using an Olympus BX53 system microscope equipped with an Olympus UC30 camera and an Olympus SZX16 stereo microscope fitted with an Olympus SC30 camera.
Two distal chondroderms were sacrificed for in-depth ultrastructural and molecular analysis. They were collected using a low-vibrational saw, μCT-scanned, washed multiple times with ultrapure (Milli-Q) water and ethanol (VWR, 96% rectapur), and stored loosely wrapped in aluminium foil prior to being treated with 8 ml 0.5 M ethylenediaminetetraacetic acid (EDTA, Panreac Applichem) at pH 8.0 in tissue culture plates (VWR). The buffer solution was exchanged on a daily basis for five consecutive days, and the soft, semi-transparent debris liberated during the demineralization process was then transferred in 50 µl portions to separate glass vials (VWR). Adhering skin was also isolated from the sediment and placed in separate vials. All samples were washed 8 times by removing all but 150 µl of the buffer solution (after all of the remaining solids had settled) and then adding 1.35 ml washing solution. Three different washing solutions were used depending on sample and subsequent analysis: for the chondroderm samples, aqueous ammonium formate (0.25 M, 5 times, followed by 0.15 M, 3 times, pH 7.0; Bioultra Sigma Aldrich) was used for time-of-flight secondary ion mass spectrometry (ToF-SIMS) and aqueous sodium chloride (0.25 M, 5 times, followed by 0.15 M, 3 times, pH 6.3; Bioxtra Sigma Aldrich) for infrared (IR) microspectroscopy, while Milli-Q water was used for the skin samples. After washing, all samples were transferred in 50 µl aliquots to silicon wafers or CaF2 windows (12 × 1 mm, Eksma Optics) depending on analysis (ToF-SIMS or IR microspectroscopy), and left to air dry in a semi-closed box.
Modern reference materials
Three deceased female harbour porpoise (Phocoena phocoena) calves (specimens 22-VLT000946, 22-VLT000947 and 22-VLT000981) were photographed and dissected at Statens veterinärmedicinska anstalt (SVA) in Uppsala, Sweden. These animals were provided as incidental fisherman bycatch in Swedish waters, and received in near-perfect condition. The flippers were removed with scalpels and knives before being transported to Lund University in containers filled with ice. Tissue samples from one of the flippers were immersed in a freshly prepared fixative solution, 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 24 h at 4 °C. The samples were then dehydrated in a graded ethanol series and embedded in epoxy resin (Agar 100, Resin kit R1031) via acetone, which was left to polymerize for 48 h at 60 °C. Semi-thin (1.5 μm) light microscopic sections were then cut with a glass knife using a Leica EM UC7 Ultramicrotome, and mounted on objective glasses. Every second section was stained with Richardson’s solution prior to examination using an Olympus BX53 system microscope equipped with an Olympus UC30 camera.
Polarized and ultraviolet light photography
Photography was performed using a Canon EOS 600D camera equipped with 18–55 mm standard lenses. The camera was mounted onto an overhead rig, and both the focal length and aperture were kept at the same settings throughout the study. Photographs were taken in a dark room using specialized (polarized and ultraviolet) lighting to illuminate the fossil. Multiple photographs were taken at a distance of ~40 cm to the specimen and then merged into a single image and distortion corrected using Adobe Photoshop (v.CC 22.3.0).
Polarized light photography was done following the recommendations by Crabb50. Two LED camera lights (Neewer) equipped with custom-fitted polarizer sheets provided continuous light. A circular polarizer filter was affixed onto the camera lens and rotated until maximum cross-polarization was achieved. Photographs were taken at an ISO setting of 100 with an exposure time of 1 to 2 s.
Ultraviolet-induced visible fluorescence photography51 was conducted using two adjacent facing 25 W Eurolite ultraviolet spotlights to illuminate the fossil. A Schott 455-nm longpass filter was taped onto a step-up ring and attached to the camera lens. Photographs were taken with an exposure time of 30 s and an ISO setting of 200.
FEG-SEM and EDX
FEG-SEM analyses were performed using two different instruments. At RISE, both untreated and demineralized samples previously analysed by ToF-SIMS were coated with a 15-nm-thick film of gold/palladium and examined in a Zeiss Supra 40VP FEG-SEM instrument at an electron energy of 2.0 keV and a working distance of ~6 mm using the standard Everhardt-Thornley type detector (SE2) and the Zeiss SmartSEM v6 software. Elemental analyses and mappings were done using an energy-dispersive X-ray microanalysis (EDX) detector from Oxford Instruments (X-Max 50, 50 mm2) at an electron energy of 15 keV and a working distance of ~8.5 mm. Collection and analysis of the EDX data were done using the Aztec software, v.3.3 and v.6.1 (Oxford Instruments Nanotechnology Tools Ltd).
At Lund University, both modern and fossil samples were coated with a 6-nm-thick layer of platinum/palladium and examined in a Tescan Mira3 High Resolution Schottky FEG-SEM fitted with both standard and in-lens secondary electron, as well as back-scattered electron, detectors at an acceleration voltage varying between 1 and 15 kV at a working distance of 3–15 mm. Elemental analyses and mappings were performed with a linked energy-dispersive spectrometer (X-MaxN 80, 124 eV, 80 mm2) from Oxford Instruments. The EDX data were processed and analysed using Aztec (v.6.1) from Oxford Instruments Nanotechnology Tools Ltd.
Transmission electron microscopy
Demineralized fossil skin was immersed in epoxy resin (AGAR 100, Resin kit R1031), which was left to polymerize at room temperature for 72 h, followed by 48 h at 60 °C. Ultra-thin (50 nm) sections were cut using a Leica EM UC7 Ultramicrotome equipped with a diamond knife, and mounted on pioloform-coated copper grids without further treatment or staining. All sections were examined in a JEOL JEM-1400 PLUS TEM at 100 kV. Micrographs were recorded with a JEOL Matataki CMOS camera using TEM Centre for JEM-1400 Plus software.
X-ray computed tomography
The blocks containing the proximal portion of SSN8DOR11 were assembled and embedded in sand, and then scanned in a Siemens Definition Flash CT scanner (Siemens Healthineers). The rock slabs were examined at 140 kV, using a dual tube (flash scan) with a tube current of 950 mA and a pitch of 0.35 to enable sufficient signal through the entombing sedimentary matrix. Images were then reconstructed using a medium soft filter and reviewed as 1 mm slices.
X-ray computed microtomography
X-ray computed microtomography was performed on two distal chondroderms (see ‘Fossil material’) using a ZEISS Xradia 520 Versa 3D X-ray microscope (4D Imaging Lab, Division of Solid Mechanics, Lund University, Sweden). The chondroderms were scanned with a source voltage of 80 kV, and the manufacturer-supplied Le4 source filter was applied to reduce beam hardening effects. The subsequent tomographic reconstructions, using the ZEISS reconstructor software (XradiaReconstructorApp V11.0) with correction for the centre of rotation, provided the 3D image volume of cubic voxels with side lengths of 2.875 µm output as 16-bit tiff slices. The chondroderms were then segmented and virtually reconstructed from the scan slice data without down-sampling using the 3D Slicer 4.6.252 and Drishti 3.053 software packages.
Synchrotron radiation X-ray tomographic microscopy (SRXTM)
SRXTM was performed at the TOMCAT beamline X02DA of the Swiss Light Source (Paul Scherrer Institut, Villigen, Switzerland). Four samples collected from a single chondroderm were immersed in water to enhance the image quality, and then scanned with a beam energy of 12 keV (2–3% bandwidth). The transmitted X-ray radiation was converted into visible light using a 20-µm-thick GGG:Eu scintillator (the distance between the sample and scintillator was a few mm) and magnified with a ×20 objective. The projections were recorded with a sCMOS camera (PCO.edge 5.5). Two sets of tomographic scans were acquired: ‘slow’ scans (aimed at higher image quality) had 1,000 equiangularly distributed projections recorded during the rotation of the sample over 180°. For ‘fast’ scans (which lead to higher stability of the sample during acquisition), the number of projections was reduced to 500. The exposure time per projection was 100 ms. The data illustrated in Fig. 3h and Supplementary Video 2 were acquired using the ‘fast’ configuration. The reconstruction was made using an in-house version of gridrec54, a software based on a gridding procedure. The cubic voxel side length of the resulting tomograms was 0.33 µm. The tomographs (as 16-bit .tiff images) were processed and analysed using the Voxler 3 software.
In addition, two skin samples were measured on the I12-JEEP beamline55 at the Diamond Light Source using a 90 keV monochromatic beam with a high-resolution imaging camera equipped with a scintillator (Crytur), a custom radiation resistant visible light optical module (SILL Optics) with a resolution of 3.24 µm × 3.24 µm per pixel, and the commercial visible light sCMOS sensor, PCO.edge 5.5 (PCO imaging, now Excellitas). The samples were scanned at 2,400 angles with an angular resolution of 0.075 degrees per step. The tomographic reconstructions were performed using the SAVU system56. The reconstructed data were segmented and analysed with the 3D Slicer software.
Time-of-flight secondary ion mass spectrometry
ToF-SIMS analyses were carried out in three instruments (all by IONTOF): a TOFSIMSIV and M6 located at RISE in Borås, Sweden, and a TOFSIMS 5 at Chalmers Materials Analysis Laboratory (CMAL), Chalmers University of Technology, Sweden. Negative- and positive-ion data were acquired using \({{\rm{Bi}}}_{3}^{+}\) primary ions (25–30 keV) and low energy electron flooding for charge compensation. High-mass-resolution data were obtained in the bunched mode (m/Δm = 5,000–10,000; lateral resolution, 2–5 µm; 0.1–0.2 pA pulsed current) and high-image-resolution data were measured in the fast-imaging mode (lateral resolution, 0.1–0.5 µm; m/Δm = 300; approximately 0.04 pA pulsed current). The fossil spectra were compared against spectra acquired for various reference materials, including Sepia officinalis eumelanin, calcium carbonate and hydroxyapatite (all from Sigma-Aldrich). Collection and analysis of the ToF-SIMS data were done using the SurfaceLab software v.6.7, v.7.1 and v.7.3 (IONTOF).
IR microspectroscopy
Hyperspectral images were recorded at the SMIS beamline at Synchrotron SOLEIL, France, with an Agilent Cary 620 microscope coupled to a Cary 670 FTIR spectrometer (Agilent Resolutions Pro 5.3.0 software, Agilent Technologies), using the internal thermal source. The microscope was equipped with a 128 × 128 pixels Lancer MCT Focal Plane Array detector. All images were recorded in reflection and high magnification mode with a ×15 objective, giving a field of view of 141 × 141 µm and a projected pixel size of 1.1 × 1.1 µm². A total of 512 scans were collected per pixel at 8 cm−1 spectral resolution, and processed using Kramers–Kronig transforms to extract absorption coefficients.
Hyperspectral images were also recorded at the Centre for Environmental and Climate Science, Lund University, Sweden, with a Hyperion 3000 IR microscope (operated in transmission mode) coupled to a Tensor 27 spectrometer (Bruker OPUS 8.5 software). The images were collected using a 64 × 64 pixel MCT Focal Plane Array detector. The distance between the detector elements was 2.3 µm. In total, 1,024 scans were collected per pixel at 4 cm−1 spectral resolution.
The chemical images were generated using the Quasar 1.7.0 package57 and superimposed onto visible microscopy images. Heat maps were produced using the baseline corrected area of peaks of interest as indicated in Extended Data Fig. 6c,d.
Computational fluid dynamics
Owing to the very low Mach number (M = 0.001) and because acoustic fluctuations scale with the square of M, direct computation of the noise by solving a compressible set of Navier–Stokes equations is not possible. Therefore, we used a hybrid computational hydroacoustic approach, where the flow was calculated by solving an incompressible set of Navier–Stokes equations, and the sound propagation estimated from an acoustic analogy. All computations were done using the pimpleFoam solver, which is part of the OpenFOAM package (an open-source computational fluid dynamics software). The cfMesh utility was employed to generate a hex-dominant, unstructured mesh with multiple levels of local refinements in the vicinity of our virtual flipper section, as well as in the wake region of this geometry. The need to compute acoustic fluctuations inhibits the use of steady flow solvers. As a consequence, flow was resolved in time using large eddy simulations, with the wall-adapting local eddy-viscosity sub-grid scale model to account for turbulent fluctuations. The time evolution of the acoustic pressure was computed using the Curle acoustic analogy58, which is an extension of the Lighthill acoustic analogy to account for the presence of solid surfaces. Because the acoustic wave propagation is not numerically resolved but instead analytically integrated at desired virtual microphone locations, these can be placed outside of the region covered by the flow solver. Post-processing was done using ParaView 5.7.0, Grace 5.1.25 and a custom tool59 based on the open-source fftw3 library.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.