Cell lines and animals
The HaCat, mouse hepatoma (AML-12), mouse embryonic fibroblast (3T3-L1) and mouse skeletal muscle (MSMC) cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences. The cells expressing green fluorescent protein (HaCatGFP cells) were established by lentivirus transfection of GFP plasmids into HaCat cells according to the manufacturer’s protocol (Shanghai Genechem). All of the cell lines were incubated in a nutritious DMEM medium containing 10% FBS and 1% (v/v) penicillin–streptomycin at 37 °C with 5% CO2.
Male C57BL/6J mice (aged 6–8 weeks, 25 g) and female Sprague–Dawley (SD) rats (aged 8–12 weeks, 200 g) were purchased from Shanghai SLAC Laboratory Animal. The mice and rats were housed in the Laboratory Animal Center of Zhejiang University under specific-pathogen-free conditions. Guangxi Bama-minipigs (male, aged 6 months, 35–40 kg) were purchased from Shanghai Jiagan Laboratory Animal and housed in the Laboratory Animal Center of Zhejiang University. The animals were fed with a standard diet and maintained under a 12 h–12 h light–dark cycle, with free access to water throughout the experiment unless otherwise specified. The ambient environment was controlled at 20–26 °C and 50–70% relative humidity. All of the animal experiments were carried out according to the protocols approved by the Institutional Animal Care and Use Committee of Zhejiang University (ZJU20250230).
Experimental materials
Unless otherwise indicated, all materials were purchased from Sinopharm Chemical Reagent. Dichloromethane (CH2Cl2) and tetrahydrofuran (THF) were distilled over calcium hydride (CaH2) or treated with a 4 Å molecular sieve. Trifluoroacetic acid (TFA), 2,2′-azobis(2-methylpropionitrile) (AIBN), 2-(dimethylamino) ethyl methacrylate (DMA), N-Boc-ethylenediamine, N,N-diisopropylethylamine (DIPEA), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) and 1-hydroxybenzotriazole (HOBT) were purchased from Energy Chemical. Fluorescein isothiocyanate (FITC), sulfo-cyanine3 succinimidyl ester (Sulfo-Cy3-NHS) and sulfo-cyanine5 succinimidyl ester (Sulfo-Cy5-NHS) were purchased from Lumiprobe. Human recombinant insulin (29 U mg−1, I8830) was purchased from Solarbio Science & Technology. NBD-C6-HPC was purchased from J&K Scientific. 4-Cyano-4-[[(dodecylthio) carbonothioyl] thio]pentanoic acid (CTA) was purchased from Aladdin. 2-Iminothiolane hydrochloride and STZ were purchased from Macklin. Gold nanoparticles (AuNPs, 5 nm) were purchased from Feynman Biotechnology. N-Succinimidyl 15-azido-4,7,10,13-tetraoxapentadecanoate (NHS-PEG4-N3) was purchased from Aikon. N-succinimidyl 4-[(5-aza-3,4:7,8-dibenzocyclooct-1-yne)-5-yl]-4-oxobutyrate (DBCO-NHS) was purchased from New Research Biosciences.
Synthesis of N-[2-(N-tert-butoxycarbonylamino)]ethyl-4-(dodecyltrithio-carbonate)-4-cyanopenteramide (N-Boc-CTA)
CTA (2 mmol), EDC·HCl (2.5 mmol) and HOBT (2.5 mmol) were dissolved in 20 ml dry CH2Cl2 and stirred at room temperature for 4 h. N-Boc-ethylenediamine (2.5 mmol) and DIPEA (2.5 mmol) were dissolved in CH2Cl2 (10 ml) and added dropwise to the above solution to continue the reaction overnight at room temperature. The mixture was successively washed twice with a saturated solution of Na2CO3, a 0.1 M HCl solution and a saturated brine solution, and then dried over MgSO4. The crude product was passed through a column packed with silica gel using a mobile phase of n-hexane and ethyl acetate mixture (1:1). A yellow solid was obtained.
Synthesis of Boc-amino-terminated poly[2-(N,N-dimethylamino)ethyl methacrylate] (N-Boc-PDMA)
DMA (30 mmol), N-Boc-CTA (0.35 mmol) and AIBN (0.07 mmol) were dissolved in THF (30 ml) in a Schlenk flask and bubbled with dry N2 for 20 min. The reaction was carried out at 65 °C for 12 h. After terminating the polymerization by opening the flask, the solution was concentrated and poured into cold n-hexane. The precipitated N-Boc-PDMA was isolated and then dried under a vacuum.
Synthesis of Boc-amino-terminated poly[2-(N-oxide-N,N-dimethylamino)ethyl methacrylate) (N-Boc-OP)
N-Boc-PDMA (0.5 g) was dissolved in 5 ml of 30% hydrogen peroxide (H2O2) solution. The mixture was stirred at room temperature for 4 h and then dialysed against deionized water to remove the unreacted H2O2 completely. N-Boc-OP was obtained after lyophilization.
Synthesis of OP-NH2
N-Boc-OP (500 mg) dissolved in 5 ml CH2Cl2 and 5 ml TFA was added dropwise under ice cooling. This solution was stirred for 2 h at room temperature. The reaction solution was evaporated to remove TFA, vacuum-dried and then dissolved in deionized water. The pH of the solution was adjusted to 7.4 with sodium hydroxide solution (1 M) and then dialysed against deionized water. OP-NH2 was obtained after lyophilization.
Synthesis of OP-DBCO
OP-NH2 (30 mg) was dissolved in 3 ml of PBS (pH 7.4), and DBCO-NHS (3 mg) in 2 ml of DMF was added. The mixture was stirred at room temperature for 4 h and then dialysed sequentially against a water-DMF mixture (3:2 (v/v)) and water. The product, OP-DBCO, was obtained by lyophilization. PEG-DBCO was prepared according to the same procedure.
Synthesis of OP–I
The lysine residue on insulin was introduced with an azide group using N3-PEG4-NHS. Insulin (21.6 mg) was dissolved in 5 ml of 0.1 M Na2CO3 (adjusted to pH 10), and N3-PEG4-NHS (1.44 mg, 10 mg ml−1 in DMSO) was added at an azido-to-amine ratio of 1:3. The mixture was stirred at room temperature for 4 h, and the azide-modified insulin (insulin-N3) was purified using preparative HPLC (prep-HPLC). For OP–I synthesis, insulin-N3 (2 mg, 0.29 μmol) and OP-DBCO (3.9 mg, 0.88 μmol) were dissolved in PBS (pH 7.4) and stirred at room temperature for 4 h. The product, OP–I, was purified using prep-HPLC. PEG–I was synthesized similarly.
Labelling OP, OP–I, PEG–I and insulin with FITC, Cy3 or Cy5
FITC, Sulfo-Cy3-NHS or Sulfo-Cy5-NHS solutions in DMSO (5 mg ml−1) were added dropwise with gentle stirring to OP-NH2, PEG-NH2, insulin or their conjugates (OP–I or PEG–I) dissolved in PBS (10 mg ml−1, pH 7.4) at a dye-to-insulin molar ratio of 1:1. The reaction was carried out overnight at room temperature in the dark. The labelled polymers, insulin and conjugates were purified using Sephadex G-25 resin to remove the unreacted dye and then lyophilized. The products were stored in the dark at 4 °C for further use.
RP-HPLC analysis
The RP-HPLC analysis was performed using a 1260 binary HPLC pump equipped with a ZORBAX SB-C18 column (5 μm, 4.6 × 250 mm) and a 1260 infinity II variable wavelength detector set at 280 nm. The mobile phase consisted of water with 0.1% TFA (phase A) and acetonitrile with 0.1% TFA (phase B). The flow rate was set at 1 ml min−1. The elution gradient was set as follows: 30% to 40% B, 0–5 min; constant 40% B, 5–10 min; 40% to 100% B, 10–11 min; constant 100% B, 11–16 min; 100% to 30% B, 16–17 min; constant 30% B, 17–22 min.
Stability of OP–ICy5
OP–ICy5 (0.4 mg) was incubated in 200 μl of DMEM culture medium with 10% FBS at 37 °C. The solution was sampled at 0 h, 6 h and 12 h for RP-HPLC detection (n = 3 per timepoint), as described above, except that an Agilent 1260 Infinity II fluorescence detector (640 nm excitation, 660 nm emission) was used. The mobile phase remained the same, but the elution gradient was as follows: 0%–100% B, 0–15 min; isocratic at 100% B, 15–20 min; 100%–0% B, 20–21 min; and isocratic at 0% B, 21–26 min. OP–ICy5 had a retention time of 8.8 min. The target peak integral area was quantified, confirming its structural stability even after 12 h.
Prep-HPLC for purification
Prep-HPLC was performed using the Waters Prep 150 LC system equipped with a Pursuit 5 C18 column (250 × 21.2 mm) and a Waters 2489 UV/VIS detector set at 214 nm and 280 nm. The mobile phases included water with 0.1% TFA (phase A) and acetonitrile with 0.1% TFA (phase B). The elution gradient and flow rates were set as follows: 20% to 40% B with flow rates increasing from 10 to 15 ml min−1, 0–10 min; constant 40% B with a constant flow rate of 15 ml min−1, 10–20 min. The retention times were 11.8 min for insulin-N3, 10.1 min for OP–I and 12.8 min for PEG–I.
Gel-permeation chromatography
The Shimadzu Prominence Plus LC-20AD LC system was equipped with two columns connected in series (PL aquagel-OH MIXED-H and PL aquagel-OH 30), a refractive index detector and a UV/VIS detector. The mobile phase was prepared by mixing acetic acid (100 ml), acetonitrile (150 ml) and deionized water (200 ml). The pH was then adjusted to 2.3 using a concentrated ammonia solution, and the final volume was brought to 500 ml with deionized water. The flow rate was 0.5 ml min−1, and the column temperature was 40 °C.
Circular dichroism spectroscopy
Far UV circular dichroism spectra were recorded at 37 °C on the JASCO J-815 spectropolarimeter. Quartz cuvettes with a path length of 1 mm were used. Each spectrum was an average of four scans recorded from 260 to 200 nm at 1 nm steps.
MALDI-TOF MS analysis
All MALDI-TOF MS analyses were performed on a Bruker Autoflex maX TOF/TOF mass spectrometer (Bruker), equipped with a modified Nd:YAG laser in positive-ion mode; data acquisition was conducted using the Bruker flexControl 3.4 software. α-Cyano-4-hydroxycinnamic acid was used as the matrix. As OP did not generate detectable signals in mass spectra owing to its zwitterionic N-oxide structure, the OP–I was then reduced to PDMA-I by bis(pinacolato)diboron39 for analysis. Ions were extracted into the mass spectrometer in reflection mode using an extraction potential of 20 kV using a high-mass detection method.
Zeta potential measurements
The solutions were prepared by dissolving OP (0.1 mg ml−1) or OP–I (0.04 mg ml−1) in HEPES buffers at varied pH values (10 mM). A Nano ZS Zetasizer (Malvern Instruments) was used to measure the zeta potentials using a 4 mW 633 nm He-Ne laser at 25 °C.
Synthesis of OP–AuNPs
OP-NH2 (60 mg) was dissolved in 6 ml of PBS (pH 8.0), and 2-iminothiolane hydrochloride (Traut’s reagent, 10 mg ml−1) was added. The mixture was stirred at room temperature for 4 h and dialysed against water (MWCO 1 kDa) to remove the unreacted components. The product was lyophilized to obtain OP-SH. OP-SH (1 mg) was added to a solution of AuNPs (50 μg ml−1) and stirred at 4 °C for 12 h. The resulting OP–AuNPs were purified by centrifugation at 19,000g for 10 min at 4 °C and then resuspended in water.
In vivo skin permeation of OPCy5, OP–IFITC, OP–ICy5 and their mixtures
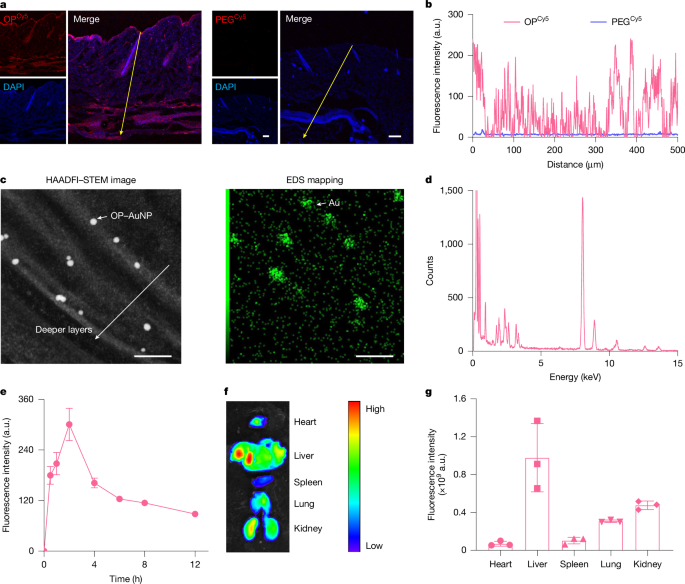
The dorsal skin of male C57BL/6J mice was exposed using depilatory cream and cleaned using PBS. A diffusing cell of 1.13 cm2 was attached onto the dorsal skin. OPCy5, OP–IFITC, PEG–IFITC, insulinFITC, OP–ICy5 (FITC or Cy5-equivalent dose, 10 μg ml−1; 0.2 ml per mouse) or their physical mixture was injected into the cell. After timed topical administration, the mice were euthanized. The treated skin sites were washed and dissected. The entire skin samples were carefully washed three times with PBS, fixed with 4% paraformaldehyde (PFA) solution and sectioned into 10-μm-thick slices using a cryostat (UV800, Leica Microsystems). 4′,6-Diamidino-2′-phenylindole (DAPI) was used to stain and label the nuclei of skin tissues, and BODIPY was used to stain and localize subcutaneous fat deposits. Fluorescence images were taken using CLSM with excitation at 405 nm for DAPI, 488 nm for BODIPY or FITC, and 640 nm for OP–ICy5. The CLSM images were analysed using ImageJ.
OPCy5 solution (0.2 ml, Cy5-equivalent dose, 10 μg ml−1) was applied to the mice as described above. Blood samples (200 μl) were collected from the orbit venous plexus at timed intervals, and their Cy5 fluorescence intensities were measured using a microplate spectrophotometer with excitation at 640 nm. The main organs of the mice, including the heart, liver, spleen, lung and kidneys, were also collected at timed topical administration of OPCy5, and their fluorescence intensities were measured using the IVIS Spectrum imaging system (IVIS Lumina XRMS Series III, PerkinElmer).
OPCy5 was dispersed in a cream (water-in-oil, Aquaphor; Cy5-equivalent dose, 10 μg ml−1). The OPCy5 cream was topically applied on the minipig abdominal skin (cream volume, 10 ml; application area, 100 cm2) for 4 h. The minipigs were euthanized, and the treated skin sites were dissected and sectioned into slices, as mentioned above. Fluorescence images were taken using CLSM with excitation at 405 nm for DAPI and 640 nm for OP–ICy5. The CLSM images were analysed using ImageJ.
Biological half-life of OP–I
Male C57BL/6J mice were randomly assigned to groups (n = 3 for each group). The solution (0.1 ml; Cy5-equivalent dose, 0.1 mg per kg) of insulinCy5 or OP–ICy5 was injected through the tail vein. Blood samples were collected from the orbital venous plexus at timed intervals. The fluorescence intensity was quantified using the IVIS Spectrum system. The insulin concentration was also measured using human insulin ELISA kits (Elabscience). The half-lives of insulin and OP–I were calculated using DAS2 software based on the plasma concentration data.
HAADFI–STEM-EDS analysis of OP–AuNP-treated skin cryosections
C57BL/6J male mice were topically administered with 0.2 ml OP–AuNPs (OP-equivalent dose, 0.5 mg ml−1). After 4 h, the mice were anaesthetized. The skin at the treated site was dissected and fixed overnight at 4 °C. The samples were trimmed into small sections and then treated with 1% osmium tetroxide (diluted in 100 mM cacodylate buffer) for 2 h, dehydrated with ethanol and acetone, and embedded in Spurr resin. Then, 10-μm-thick slices were sectioned (LKB 11800 Pyramitome) and stained with toluidine blue to select the desired regions. The ultrathin sections (50–100 nm) were prepared using an ultramicrotome (UC7, Leica).
HAADFI STEM-EDS analysis of the skin cryosections was performed on a field emission transmission electron microscope (JEOL JEM F200) at an accelerating voltage of 200 keV equipped with an EDS detector.
Cellular uptake
AML-12 cells, 3T3-L1 differentiated adipocytes and MSMC cells were seeded into confocal dishes at a density of 1 × 105 cells per ml and incubated for 24 h. The cells were treated with OP–ICy5 (Cy5-equivalent dose, 1 μg ml−1) for 4 h. After incubation, the nuclei were stained with Hoechst 33342 (2 μM) for 20 min. The cells were washed twice with PBS and imaged using CLSM at an excitation wavelength of 405 nm for Hoechst 33342 and 640 nm for Cy5.
SPR analysis of binding affinity
SPR experiments were performed on a Biacore X100 instrument (Cytiva) with data acquisition using Biacore X100 system control software (v.2.0.1.201). The CM5 chip sensor (GE Healthcare) was used in this study. Anti-His antibodies were immobilized on the surface of the CM5 chip sensor according to the manufacturer’s instructions. His-tag-ECD-IR/IGF1R protein (Sino Biological) was then injected over the anti-His antibody-coated surface of the CM5 chip sensor at a specific concentration and flow rate. The binding affinities of insulin and OP–I were evaluated by injecting various concentrations of insulin (9.75, 19.5, 39.0, 78.0 and 156 nM) or OP–I (4.06, 8.13, 16.3, 32.5 and 65.0 nM) in HEPES running buffer (pH 7.4) over the His-tag-ECD-IR/IGF1R-coated surface under a single-cycle kinetics mode. Each cycle included a 180 s binding phase and a 300 s dissociation phase. The dissociation equilibrium constant (Kd), association rate constant (kon) and dissociation rate constant (koff) were determined using the BIA evaluation software (v.2.0.1.201). The t1/2 values, which define the residence time, were determined using the formula ln2/koff.
Western blotting for insulin signalling analysis
Mice were injected subcutaneously with PBS or insulin (insulin-equivalent dose, 5 U kg−1), or topical administration with OP–I (insulin-equivalent dose, 116 U kg−1; concentration, 0.5 mg ml−1; volume, 0.2 ml; diffusing area, 1.13 cm2). The mice were euthanized, and their skeletal muscle tissues were collected at 1 h after subcutaneous injection or 4 h after topical administration. Muscle samples were weighed and lysed in radioimmunoprecipitation buffer (Sigma-Aldrich) containing protease and phosphatase inhibitors (Thermo Fisher Scientific) and incubated on ice for 30 min. The lysates were centrifuged at 20,000g for 20 min at 4 °C and quantified using the BCA protein assay. The proteins were resuspended in Laemmli sample buffer with 2.5% 2-mercaptoethanol, denatured at 95 °C for 5 min, separated by SDS–PAGE and transferred to polyvinylidene fluoride membranes. The membranes were blocked in Tris-buffered saline with 0.5% Tween-20 (TBST) and 5% BSA for 1 h, followed by overnight incubation at 4 °C with primary antibodies against phosphorylated IRβ/IGF1Rβ (1:1,000), phosphorylated AKT-Thr308 (1:1,000) or GAPDH (1:5,000). After washing three times with TBST, the membranes were incubated with horseradish-peroxidase-conjugated secondary antibodies (HRP-labelled goat anti-rabbit IgG (H+L) or HRP-labelled Goat Anti-Mouse IgG (H+L)) at a dilution of 1:1,000 at room temperature for 1 h. Signal detection was performed using Immobilon Forte chemiluminescence substrate on the ChemiScope 3600 Mini Imaging System (Clinx Science Instruments). The membranes were next incubated in stripping buffer (BL526, Biosharp) at room temperature with gentle agitation for 15 min, followed by extensive washing with TBST, and then overnight incubation at 4 °C with primary antibodies against IRβ and AKT. The membranes were processed as previously described, including incubation with HRP-conjugated secondary antibodies and signal detection through chemiluminescence.
OP–I permeation in 3D skin equivalent EpiKutis
The 3D skin model (EpiKutis) was fabricated according to a previously reported method40. In brief, keratinocytes (5 × 105) were seeded onto the permeable membrane of a Transwell chamber, cultured at 37 °C in a 5% CO2 atmosphere for 2 days and then cultured at the air–liquid interface for 8 days with daily medium replacement. The complete EpiKutis 3D model was obtained and used as a skin model for investigating OP–I skin permeability. OP–I, PEG–I or insulin solution (insulin-equivalent dose, 0.5 mg ml−1; 0.2 ml) was added to the donor compartment, and 0.4 ml fresh medium was added to the receiving compartment. The temperature was maintained at 37 °C. At timed intervals, 50 μl solution was withdrawn from the receiving compartment and an equal volume of fresh medium was added. The insulin concentration was quantified by ELISA kits. The unit conversion was calculated according to the following formula, and the cumulative amount of insulin permeating per unit area of the model skin (Qn) was calculated:
$${Q}_{n}=\frac{{C}_{n}{V}_{{\rm{r}}}+{\sum }_{i=1}^{n-1}{C}_{{\rm{i}}}{V}_{{\rm{s}}}}{A}$$
Where, Qn is the cumulative amount of insulin permeating per unit area (μg cm−2); Cn is the insulin concentration in the receiver cell at sampling timepoint t (μg ml−1); Ci is the insulin concentration of the receiving liquid at the intermediate point (μg ml−1); Vr is the volume of the receiving pool (0.4 ml); Vs is the volume of the sampled receiving solution (50 μl); and A is the effective transmission area of the EpiKutis (0.081 cm2).
The steady-state flow rate (Jss) was obtained as the slope of the curve of Qn as a function of time. The apparent permeability coefficient (Kp) was calculated by the following formula:
$${K}_{{\rm{p}}}=\frac{{J}_{\mathrm{ss}}}{{C}_{0}}$$
Where C0 is the initial concentration of insulin or its conjugate in the donor cell.
EpiKutis (0.081 cm2) was incubated with 0.2 ml OP–IFITC, PEG–IFITC or insulinFITC (FITC-equivalent concentration, 1 μg ml−1) for 4 h, then washed three times with PBS, stained with DAPI and finally imaged using CLSM with the z-stack tomoscan model at a 10-μm interval from the bottom to top of the 3D skin model with excitation at 405 nm for DAPI and 488 nm for FITC.
Skin penetration analysis by intravital two-photon microscopy
The mice were topically treated with OP–ICy5, as described above. The skin was mounted between a coverslip and a sliding glass for two-photon imaging analysis. The laser wavelength for two-photon excitation was 480 nm, and the laser power delivered to the skin sample was 90 mW. Sequential z-stack images were captured at 3-μm intervals from the skin surface until the fluorescence signal became undetectable. The xz-axis orthogonal view of the SC layers was reconstructed using volume viewer plugins in ImageJ.
Subcutaneous lymphatic vessel co-localization
OP–ICy5 (Cy5-equivalent dose, 0.2 ml of 10 μg ml−1) was topically applied on the dorsal skin of SD rats. After 4 h, the tissue at the application site was excised and frozen-sectioned. The lymphatic vessels were immunostained using LYVE-1AF488 (1:1,000), and the nuclei were counterstained with DAPI. Subsequently, the distribution of OP–ICy5 within the lymphatic vessels was examined using CLSM.
Skin retention of OP–ICy5
After 4 h of topical application with OP–ICy5 on the dorsal skin of C57BL/6J mice (Cy5-equivalent dose, 10 μg ml−1; 0.2 ml per mouse), the OP–ICy5 solution was removed and the treated skin site was gently washed three times with PBS. Subsequently, the mice were euthanized at 4 or 8 h after removal of the OP–ICy5 solution. The fluorescence intensity in the skin was imaged using the IVIS Spectrum imaging system and CLSM.
In vivo skin permeation of OP–ICy5 in minipigs
The OP–ICy5 cream (Cy5-equivalent dose, 10 μg ml−1) was topically applied onto the minipigs’ abdominal skin (cream volume, 10 ml; application area, 100 cm2). After 4 h, the minipigs were euthanized, and the treated site skins were washed three times with PBS, dissected, fixed with 4% PFA and sectioned into slices, as described above. The slices were stained with DAPI to label the cell nuclei and with NBD-C6-HPC to label the SC lipids and the cell membranes in the viable epidermis. Fluorescence images were taken using CLSM with excitation at 405 nm for DAPI, 488 nm for NBD-C6-HPC and 640 nm for OP–ICy5. The CLSM images were analysed using ImageJ.
OP–ICy5 biodistribution in mice
Male C57BL/6J mice were randomly grouped (n = 3 mice). Each mouse was topically applied with 0.2 ml OP–ICy5, PEG–ICy5 or insulinCy5 solution (Cy5-equivalent dose, 10 μg ml−1) on the mouse dorsal skin for 4 h, and then the solution was removed. InsulinCy5 was administered subcutaneous to the mice in the control group (Cy5-equivalent dose, 25 μg per kg). The mice were euthanized at timed intervals. The main organs and tissues, including the heart, liver, spleen, lung, kidneys, adipose tissues (including brown adipose tissue,subcutaneous white adipose tissue and visceral white adipose tissue), and skeletal muscles were collected, and their fluorescence intensities were measured using the IVIS Spectrum imaging system.
In vivo studies using STZ-induced diabetic mice
Type 1 diabetic mice were established through intraperitoneal delivery of STZ (150 mg per kg in 10 mg ml−1 disodium citrate buffer, pH 4.5) into healthy male C57BL/6J mice (aged 6–8 weeks, ~25 g). BGLs were monitored, and the mice with BGLs exceeding 300 mg dl−1 were considered diabetic. Diabetic mice with fasting BGLs within the 300 to 600 mg dl−1 range were selected for the experiments. BGLs were measured from tail-vein blood (around 3 μl) using a calibrated Sinocare glucose meter.
A diffusion cell (diffusing area: 1.13 cm2) containing insulin, OP–I or PEG–I (insulin-equivalent dose, 116, 58 or 29 U kg−1; concentration, 0.5 mg ml−1; volume, 0.2, 0.1 or 0.05 ml per mouse) or the mixture of OP and insulin (OP + insulin; insulin-equivalent dose, 116 U kg−1, 0.5 mg ml−1; 0.2 ml per mouse) was applied on the dorsal or abdominal skin of STZ-induced diabetic mice. For the positive control group, mice were administered insulin subcutaneously at a dose of 5 U kg−1. BGLs were measured at timed intervals. The skin insulin concentrations and plasma insulin concentrations in the blood samples (25 μl) collected from the tail vein were quantified using ELISA kits according to the manufacturer’s protocol. The diabetic mice were subjected to a fasting period of 12 h during the experimental procedures.
The repeatability of OP–I’s hypoglycaemic effect was assessed by applying OP–I (insulin-equivalent dose, 116 U kg−1, 0.5 mg ml−1; 0.2 ml per mouse) topically onto the same diabetic mice over three consecutive days. Plasma insulin concentrations were measured at timed intervals using ELISA.
For the IPGTT experiment, diabetic mice were topically applied with OP–I (insulin-equivalent dose, 116 U kg−1, 0.5 mg ml−1; 0.2 ml per mouse); then, 1 h later, they were intraperitoneally injected with 0.2 ml of glucose solution (1.5 g per kg). BGLs were monitored over time. The diabetic mice were subjected to a fasting period of 3 h during the experimental procedures.
In vivo studies using STZ-induced diabetic minipigs
Guangxi Bama minipigs (aged 6 months; weight, 35–40 kg) were infused with STZ in freshly prepared disodium citrate buffer (75 mg ml−1, pH 4.5) at a 150 mg per kg dose within 10 min and then maintained for recovery. The glucose levels were monitored using CGMS15 (Dexcom G4 Platinum Continuous Glucose Monitor System, Dexcom). A BGL that is constantly higher than 250 mg dl−1 indicates the successful establishment of the insulin-deficient diabetic minipig model.
OP–I, PEG–I or native insulin was dispersed in the cream (water-in-oil) at an insulin-equivalent concentration of 1 mg ml−1, as described above. The cream was topically applied onto the minipig abdominal skin at an insulin-equivalent dose of 40 mg or 10 mg (n = 3 for each group). The area with the cream was wrapped with plastic film. The BGLs of minipigs were continuously monitored using the CGMS. The diabetic minipigs were subjected to a fasting period of 12 h during the experimental procedures.
The skin samples were excised from the administration skin site at the end of the experiment. The samples were processed for sectioning and stained with haematoxylin and eosin and terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL). Moreover, control skin samples were collected from healthy minipigs in the same anatomical region without treatment.
SC sample collection and observation
The procedure was performed according to a previously reported method34. At 4 h after topical application of OP–ICy5, PEG–ICy5 or insulinCy5 (Cy5-equivalent dose, 10 μg ml−1; 0.2 ml per mouse), the treated sites were carefully washed three times with PBS and dried. Double-sided adhesive tape (Scotch 3M) was pressed onto the skin surface for 2 s and then peeled off in the longitudinal direction. The middle part of the tape was fixed on a microscopy slide, stained with NBD-C6-HPC and then observed under CLSM at 488 nm excitation for NBD-C6-HPC and 640 nm excitation for Cy5.
FTIR spectrometry
PBS (1 ml), OP (0.5 mg ml−1 in 1 ml PBS), OP–I (0.5 mg ml−1 in 1 ml PBS) or oleic acid (50 mg ml−1 in 1 ml propylene glycol) was evaluated using RYJ-12B Franz diffusion cells with male C57BL/6J mouse skin. Mouse skin samples (3 cm × 3 cm) were fixed in glass holders with a 2.2 cm2 circular permeation area, mounted in Franz cells with the epidermal side up, and the acceptor compartment (8 ± 0.5 ml) was filled with pH 7.4 PBS. The setup was maintained on a magnetic stirrer in a water bath at 37 ± 0.5 °C. After incubating for 24 h with the test compounds, skin samples were gently washed with PBS and analysed using a Thermo Fisher Scientific Nicolet iS50 FTIR spectrometer. Spectra were acquired by co-adding 128 scans at 4 cm−1 resolution over the frequency range of 4,000–650 cm−1.
MD simulations
The insulin (PDB: 1AI0 (ref. 41), chain I) and its receptor (IR, PDB: 6SOF (ref. 42)) structures were obtained from the Protein Data Bank (PDB). The structures of OP and OP–I were constructed using Avogadro software43. OP was set to have 32 repeating units. The force-field parameters of OP and OP–I were obtained from the Paramchem webserver44 and CGenFF45. The SC lipid membrane, composed of an equimolar mixture of ceramide, cholesterol and free fatty acids, was generated by the membrane builder CHARMM-GUI46,47. The CHARMM3648 force field was used to model the insulin and SC lipids. At pH 5.5, 20% of the N-oxide groups of OP were assumed to be protonated, whereas 50% of the fatty acids were considered to be deprotonated. At pH 7.0, OP was zwitterionic, while all fatty acids were deprotonated. Each system was solvated in an 11.4 × 11.4 × 16.1 nm3 water box with around 219,000 atoms. To study the binding interactions of insulin or OP–I with IR, the respective system was solvated in a 23.0 × 23.0 × 23.0 nm3 water box containing around 1,225,000 atoms. To study the interactions of multiple insulin, OP or OP–I molecules, the respective system was solvated in a 14.7 × 14.7 × 14.7 nm3 water box containing around 325,000 atoms. Water molecules were modelled by the TIP3P water model49. Na+ and Cl− ions were added to neutralize each system and bring its total ionic strength to 0 at the physiological concentration of 150 mM.
All MD simulations were carried out using the program GROMACS 2020.650,51. VMD52 was used for trajectory visualization. The covalent bonds with hydrogen atoms were constrained by the LINCS algorithm53, which allowed a time step of 2 fs. The long-range electrostatic interactions were calculated using the particle-mesh Ewald method54, whereas the van der Waals interactions were calculated with a smooth cut-off of 1.2 nm. Periodic boundary conditions were applied in all directions. The NPT ensemble with semi-isotropic pressure coupling was applied with the pressure (1 bar) controlled by the Parrinello–Rahman barostat55 and the temperature (310 K) by the v-rescale thermostat56. Before production runs, the lipid system was equilibrated for 100 ns; then, 200-ns runs were conducted to monitor insulin, OP or OP–I adsorption on the SC lipids. After adsorptions, three independent 100-ns runs were further performed for insulin, OP or OP–I to monitor their diffusions on SC lipids. To analyse the binding interactions of insulin or OP–I with IR, 200-ns MD simulations were conducted for each system. To investigate the interactions of multiple insulin, OP or OP–I molecules at pH 6.0, 400-ns MD simulations were conducted for each system. In production simulations, all atoms were free to move.
The friction coefficient γ of insulin/OP/OP–I on SC lipids was derived from the Stokes–Einstein relation:
$$\gamma =\frac{{k}_{{\rm{B}}}T}{D}$$
where kB is the Boltzmann constant, T is the temperature and the diffusion coefficient D follows
$$D=\frac{\langle {r}^{2}(t)\rangle }{{k}{\rm{t}}}$$
Where k = 4 on a 2D surface, the time t and the displacement r(t) were obtained from three independent 100-ns MD runs after adsorption.
PMF analyses
The PMF results were calculated using the umbrella sampling protocol57. The PMF setups were similar to the aforementioned MD setups to estimate the energy barriers for the diffusion of insulin, OP or OP–I on the SC lipids. The total transverse distance along each representative path was 2 nm, which was divided into 20 windows with a resolution of 0.1 nm. Position restraints were applied to the SC lipids when the energy barriers were scanned. For monitoring the adsorption of insulin, OP, or OP–I on SC lipids, the simulation boxes were extended to 11.4 × 11.4 × 22.1 nm3 by adding 0.15 mM NaCl solution in the boxes. After a further 30-ns equilibration, the sampling path of each system was obtained by pulling insulin, OP or OP–I in the perpendicular (z) direction to the SC lipid membrane with a constant velocity of 0.2 nm ns−1. The total sampling distance in the z direction was 9 nm for each system, which was divided into 90 windows with a resolution of 0.1 nm. At each window, the system was first equilibrated for 5 ns, followed by a 35-ns productive umbrella sampling with a restraint force constant of 1,000 kJ mol−1 nm−2. To evaluate the binding affinity of insulin or OP–I with IR, the sampling path of each system was determined by pulling insulin or OP–I perpendicular to the IR surface at a constant velocity of 0.2 nm ns−1. The total sampling distance of 5 nm was divided into 50 windows with a resolution of 0.1 nm. At each window, the system underwent a 2-ns equilibration phase followed by a 20-ns productive umbrella sampling simulation, applying a restraint force constant of 1,000 kJ mol−1 nm−2.
VE penetration study using 3D-cultured multilayer HaCat spheroids
Multilayer cell spheroids were prepared using the hanging-drop method. HaCat cells were suspended in fresh DMEM medium (containing 0.12% (w/v) methylcellulose) at a density of 4 × 105 cells per ml. The cell suspensions (25 μl) were dropped onto the lids of the cell culture plate to form uniform droplets, and 20 ml PBS was added to the plate to keep the droplets moist. The cells were incubated for 72 h and formed dense spheroids, which were transferred to an agarose-coated (1% (w/v) in PBS) 96-well plate with one spheroid per well and incubated for another 72 h to mature. The spheroids were incubated with OP–IFITC, PEG–IFITC or insulinFITC at an FITC-equivalent dose of 1 μg ml−1 for timed intervals. The spheroids were washed with PBS and imaged using CLSM by z-stack tomoscan at 20 μm intervals from the bottom to the middle of the spheroids. The integration of FITC fluorescence density and linescan analysis were performed using Image J.
For the effects of the endocytosis and exocytosis inhibitors on the penetration of OP–IFITC, the HaCat spheroids were separately treated with PBS, wortmannin (2 μM), cytochalasin D (20 μM), monensin (20 μM), nocodazole (10 μM) or brefeldin A (10 μM) for 2 h, and then incubated with OP–IFITC (FITC-equivalent dose, 1 μg ml−1) for 4 h. The HaCat spheroids were imaged and analysed as described above.
Localization of OP–ICy5 at HaCat cell membranes
HaCat cells were plated onto glass-bottom dishes at a density of 1 × 105 cells per dish and incubated for 24 h. The HaCat cells were incubated with OP–ICy5 (Cy5-equivalent dose, 1 μg ml−1) for 6 h, 12 h or 24 h. The cell membrane was stained with NBD-C6-HPC (1 μM) for 5 min, and then the cells were washed three times with PBS and observed under CLSM. Fluorescence images were taken using CLSM with excitation at 488 nm for NBD-C6-HPC and 640 nm for Cy5.
The cells were imaged immediately at maximum projection after adding OP–ICy5 (Cy5-equivalent dose, 1 μg ml−1) for time-lapse videos.
Observation of contact-dependent direct transfer of OP–I among HaCat cells
HaCat cells were seeded into six-well plates at 1.25 × 105 or 2.5 × 105 cells per well and allowed to adhere overnight. The cells were then incubated with OP–ICy5 (Cy5-equivalent dose, 1 μg ml−1) for 12 h and then extensively rinsed with sterilized PBS and isolated. The OP–ICy5-treated HaCat cells were mixed with untreated HaCatGFP cells at the same cell density. The mixed cells were co-cultured in DMEM medium for 12 h and imaged with CLSM with excitation at 488 nm for GFP and 640 nm for Cy5. The mixed cells co-cultured in DMEM medium for 3 h, 6 h or 12 h were further isolated and analysed by flow cytometry. First, an FSC-A versus SSC-A gate was applied to exclude debris and select live cells based on forward scatter (FSC) and side scatter (SSC) characteristics. Next, a GFP gate (FL1-H) was set to include cells with fluorescence in the GFP channel, followed by establishing a Cy5 gate (FL4-H) to isolate Cy5-positive cells. Finally, an intersection gate between the GFP and Cy5 gates was used to identify dual-positive cells. Every 10,000 cells were counted to determine GFP-positive cells at the FL1 channel and Cy5-positive cells at the FL4 channel. The experiment was repeated three times independently; FlowJo (v.10.0) software was used for analysis. The transfer efficiency of OP–ICy5 to HaCatGFP cells was calculated according to the formula.
$$\mathrm{Transfer}\,\mathrm{efficiency}( \% )=\frac{{\mathrm{Number\; of\; HaCat}}^{\mathrm{GFP}+\mathrm{Cy}5}\,\mathrm{cells}}{\mathrm{Total\; counted\; number\; of}\,{\mathrm{HaCat}}^{\mathrm{GFP}}\,\mathrm{cells}}\times 100$$
Non-contact inhibition of intercellular transfer of OP–ICy5
HaCat cells were seeded onto two coverslips (1 and 2) and incubated overnight. The cells on a coverslip 1 were first cultured with OP–ICy5 (Cy5-equivalent dose, 1 μg ml−1) for 4 h, rinsed with PBS three times and then co-incubated with the coverslip 2 with untreated cells in a fresh medium for 12 h. The cells on the coverslips were washed with PBS, and the cell membrane was stained with NBD-C6-HPC (1 μM) for 5 min. The cells were imaged with CLSM at 488 nm excitation for NBD-C6-HPC and 640 nm for Cy5.
Intercellular transfer between cells on separate coverslips
Two coverslips were seeded with HaCat cells (105 each) and cultured overnight to ensure full adherence. The cells on one coverslip were incubated with OP–ICy5 (Cy5-equivalent dose, 1 μg ml−1) for 12 h and then washed three times with PBS, noted as coverslip 1. Coverslip 1 was placed on top of the other coverslip with untreated HaCat cells (coverslip 2) and pressed slightly. The coverslips were cultured together in DMEM medium for 0.5 or 1 h and then observed using CLSM for the transfer of OP–ICy5.
TIRFM imaging
HaCat cells were seeded onto a confocal dish at a density of 1 × 105 cells per well and incubated for 24 h. Subsequently, OP–ICy3 (Cy3-equivalent dose, 1 μg ml−1) was added. After 4 h, NBD-C6-HPC was added to stain the cell membrane for 5 min, and the cells were then washed three times with PBS. Time-lapse imaging of the cell membrane was conducted using the TIRF function of an Olympus IX83 microscope. ImageJ was used for image analysis.
Statistical analyses
Statistical tests were performed using Prism (GraphPad software, v.10.4.0). One-way analysis of variance was used for multiple comparisons. Unpaired Student’s t-tests were used to analyse the difference between two groups.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.