Mouse and rat models
Adult male or female C57BL/6 mice (15–25 g body weight, 8–15 weeks of age) or transgenic mice were used for all experiments. vGLUT2–Cre (016963, Jackson Laboratory), Vsx2–Cre (MMMRRC 36672, also called Chx10–Cre), ChAT–Cre, VGAT–Cre, Ai65(RCFL-tdT) (021875; Jackson Laboratory), PV–Cre (017320, Jackson Laboratory), AdvillinFlpO (a gift from V. Abraira), iDTR and Calca–Cre transgenic mouse strains were bred and maintained on a mixed genetic background (C57BL/6). Adult female Lewis rats (180–220 g body weight, 14–30 weeks of age) were used for the rat experiments. Housing, surgery, behavioural experiments and euthanasia were all performed in compliance with the Swiss Veterinary Law guidelines. Manual bladder voiding and all other animal care was performed twice daily throughout the entire experiment. All procedures and surgeries were approved by the Veterinary Office of the Canton of Geneva (Switzerland; authorization GE67).
Viral vectors and vector production
Viruses used in this study were either acquired commercially or produced at the EPFL core facility. The following AAV plasmids were used and detailed sequence information is available as detailed or upon request: AAVDJ-hSyn-flex-mGFP-2A-synaptophysin-mRuby48 (reference AAV DJ GVVC-AAV-100, Stanford Vector Core Facility), AAV9-CMV-Cre (7014, Vector Biolabs), AAV5-hSyn-eGFP (50465-AAV5, Addgene), AAV5-Syn-flex-ChrimsonR-tdT (62723, Addgene), AAV5-hSyn-DIO-hm4D49 (Gi)-mCherry (44362, Addgene; 7 × 1012 vg ml−1 or more), AAV5-CAG-flex-tdTomato (a gift from S. Arber), AAV5-hSyn-Con/Fon-eYFP (#55650, Addgene), rAAV2-EF1a-DIO-Flpo (#87306, Addgene), EnvA-ΔG-Rabies-mCherry (a gift from S. Arber) and AAV8-hSyn-dlox-TVA950-2A-EGFP-2A-oGrev-dlox-WRPE-bGHp (a gift from S. Arber).
SCI models
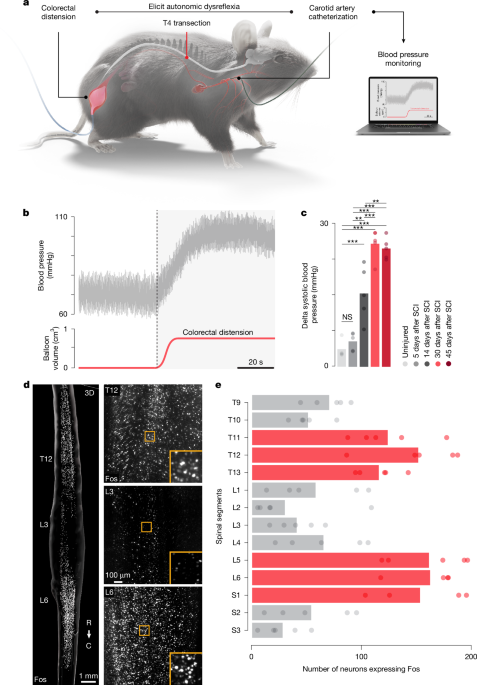
For mouse SCIs, a laminectomy was performed on the T4 vertebra to expose the T4 spinal segment. Complete transections were performed using angled microscissors. Rat SCIs were performed according to our previously published work6. In brief, a laminectomy was performed on the T3 vertebra to expose the T3 spinal segment. Following this, the rat was transferred to the Infinite Horizon (IH-0400 Impactor, Precision Systems and Instrumentation) impactor6 stage, where the T2 and T4 spinous processes were securely clamped using modified Allis forceps6. The rat was stabilized on the platform and the impactor tip (2.5 mm) was properly aligned using a 3D coordinate system moving platform. The Infinite Horizon system was set to deliver an impact force of 400 kdyn, with a 5-s dwell time6. Analgesia (buprenorphine; Essex Chemie; 0.01–0.05 mg kg−1, subcutaneously) and antibiotics (amoxicillin 200 mg per 4 ml; Sandoz; 200 mg l −1 ad libitum) were provided for 3 and 5 days after surgery, respectively. Bladders were manually expressed twice a day until the end of the experiment50.
Rodent anaesthesia use
All non-terminal experiments were conducted by anaesthetizing animals with isoflurane (initial induction at 5% and maintained on a Bain’s system at 2%). Terminal haemodynamic assessments were carried out as previously described 4 weeks after SCI. In brief, animals were anaesthetized with urethane (1.5 g kg−1, intraperitoneally)6. The depth of anaesthesia was continually monitored by assessing withdrawal reflexes and respiratory rate.
Haemodynamic monitoring
In mice, carotid artery catheterization was performed. After induction of anaesthesia, the hair on the neck of mice was shaved, and the surgical site cleaned with alcohol and betadine. The right common carotid artery was exposed and isolated from the internal jugular vein using blunt dissection. The rostral portion of the carotid artery immediately below bifurcation was permanently occluded, whereas the caudal portion of the vessel was temporarily occluded using 5-0 silk sutures. A bent-tip 30-gauge needle was used to create a small hole on top of the carotid artery. The blood pressure sensor was inserted into the carotid artery and advanced approximately 0.5 cm caudally. The catheter was then secured with two 5-0 silk sutures. In rats, the procedure for the telemeter implantation was performed according to our previously published work6. In brief, we recorded blood pressure using wireless telemeters (TRM56SP SNA and Pressure Telemeter, Kaha Sciences). A midline abdominal incision was made to expose the peritoneal cavity, followed by a blunt dissection to reach the descending aorta. The aorta was temporarily occluded using a 4-0 silk, 1–2 mm rostral to the iliac bifurcation. The pressure sensor was inserted in the aorta so that the tip was just caudal to the renal artery and fixed with a surgical mesh and biocompatible surgical glue.
Colorectal distension to induce autonomic dysreflexia
Foley Catheter Cysto-Care 1.5 ml and Foley Catheter Cysto-Care 3 ml were used for mice and rats, respectively, to perform colorectal distension to induce autonomic dysreflexia51. The catheter was inserted into the rectum and colon until the balloon was no longer exposed. During colorectal distension assessments, the balloon was inflated (up to 0.7 ml for mice and up to 2.5 ml in rats) for 60 s. Subsequent trials were only initiated after blood pressure had returned to the baseline value. For repetitive autonomic dysreflexia experiments, the balloon was inflated for 30 s and deflated for 60 s and this protocol cycled for 90 min (ref. 14).
EES implants
All the procedures have been previously detailed6,13,52,53,54,55,56. To position electrodes to deliver EES in mice, laminotomies (removal of only the connective tissue between the bones, but not the bones) were performed at T9–T10 and T12–T13 to expose the spinal cord. Teflon-coated stainless steel wires connected to a percutaneous connector (Omnetics Connector Corporation) were inserted rostrally and passed between the spinal cord and the vertebral bones to the other opening. A small part of insulation was removed and the exposed stimulation sites were positioned over T12–T13. A common ground was inserted subcutaneously. The percutaneous connector was cemented to the skull. This stimulation protocol was subsequently used for all acute and chronic experiments. In rats, the implantation of the e-dura was performed according to our previously published work6. To insert and stabilize e-dura implants into the epidural space, two partial laminectomies were performed at vertebrae levels L1–L2 and T8–T9 to create entry and exit points for the implant. The implant was gently pulled above the dura mater using a surgical suture. Electrophysiological testing was performed intra-operatively to fine-tune positioning of electrodes. The connector of the implant was secured into a protective cage plastered using freshly mixed dental cement on top of the L2–L3 vertebra. Stimulation was then delivered as previously described6. The headstage was plastered using freshly mixed dental cement on the dorsal side of the skull where three stainless steel screws were placed.
Autonomic neurorehabilitation
In mice, we delivered EES with conventional stimulation protocols6 that involved continuous EES delivered at 50 Hz with 5-ms pulses at 100–150 µA (2100 Isolated Pulse Stimulator, A-M Systems). Mice underwent autonomic neurorehabilitation consisting of EES applied for 30 min each day for 4 weeks, starting 1 week after SCI. In rats, we applied a closed-loop controlled stimulation using a proportional integral controller that adjusts the amplitude of traveling EES waves over the three haemodynamic hotspots (MATLAB). Next, we applied a +10 mmHg systolic blood pressure target from the baseline acquired at the beginning of each autonomic neurorehabilitation session6. Rats received 30 min of closed-loop controlled stimulation for 6 weeks, starting 1 week after SCI.
Neuron-specific ablations and chemogenetic manipulations
For ablation experiments with the diphtheria toxin, we used PVCre::AvilFLPo::iDTR and CalcaCre::AvilFLPo::iDTR mice. Four weeks after the SCIs (T4 spinal-level complete transection), mice received intraperitoneal injections of diphtheria toxin (D0564, Sigma) diluted in saline (100 µg kg−1) to target PVON or CalcaON neurons, respectively. Mice were tested 2 weeks post-injection. To manipulate the activity of vGLUT2ON and VGATON neurons, AAV5-hSyn-DIO-hm4D was infused (0.15 µl per injection) at two depths (0.8 mm and 0.4 mm below the dorsal surface) and separated by 1 mm in either the lower thoracic spinal cord (T11–T13) or the lumbosacral spinal cord (L5–S1) of either vGLUT2–Cre or VGAT–Cre mice before performing the SCI. To manipulate ChATON neuronal activity, AAV5-hSyn-DIO-hm4D was infused (0.15 µl per injection) at two depths (0.8 mm and 0.4 mm below the dorsal surface) and separated by 1 mm in the lower thoracic spinal cord (T11–T13) of ChAT–Cre mice before performing the SCI. To manipulate SCTHORACIC::Vsx2 or SCLUMBAR::Vsx2 neurons, AAV5-hSyn-DIO-hm4D was infused in either the lower thoracic spinal cord (T11–T13) or the lumbosacral spinal cord (L5–S1; injection depths were 0.8 mm and 0.4 mm below the dorsal surface; separated by 1 mm; 0.15 µl per injection), respectively, in Vsx2–Cre mice before performing the SCI. After 4 weeks, autonomic dysreflexia or EES-induced pressor response was assessed before and between 30 min and 45 min after intraperitoneal injections of 5 mg kg−1 clozapine N-oxide (Carbosynth, CAS: 34233-69-7; suspended in 2% DMSO in saline).
Optogenetic manipulation
To optogenetically manipulate Vsx2ON neurons, AAV5-Syn-flex-Chrimson (#62723-AAV5, Addgene; titre ≥ 5 × 1012 vg ml−1) was infused in either the lower thoracic spinal cord (T11–T13) and the lumbosacral spinal cord (L5–S1), in Vsx2–Cre mice before performing the SCI. After 6 weeks, laminectomies were made over T11/T12/T13 and L5/L6/S1 spinal segments. Five-ms pulses were delivered at 50 Hz from a 635-nm laser (LRD-0635-PFR-00100-03, LaserGlow Technologies). Laser light was delivered to the surface of the spinal cord through a fibre optic cable attached to 400 µm, 0.39 NA cannula with a 5-mm tip (Thorlabs). Optical power was set to 2.35 mW at the tip.
Spinal injections for exclusive labelling of Vsx2ON neurons
To exclusively label Vsx2ON neurons in the lumbosacral spinal cord with long-distance projections to the lower thoracic region (SCLUMBAR::Vsx2), we leveraged Boolean logic viral strategies30. Partial laminectomies were made over the T11/T12/T13 and L5/L6/S1 spinal segments of Vsx2–Cre mice. Two sets of bilateral injections of AAV5-hSyn-Con/Fon-eYFP (#55650-AAV8, Addgene; titre ≥ 1 × 1013 vg ml−1)30 were made over the L5/L6/S1 spinal segments (0.25 µl per injection) at a depth of 0.6 mm below the dorsal surface and separated by 1 mm. Two sets of bilateral injections of rAAV2-EF1a-DIO-Flpo (#87306, Addgene) were made over the T11/T12/T13 spinal segments (0.15 µl per injection) at two depths (0.8 mm and 0.4 mm below the dorsal surface) and separated by 1 mm. Animals were perfused 4 weeks later. To label lower thoracic Vsx2ON neurons (SCTHORACIC::Vsx2), two sets of bilateral injections of AA5V-CAG-flex-tdtomato were made over T11/T12/T13 spinal levels (0.15 µl per injection) at two depths (0.8 mm and 0.4 mm below the dorsal surface) and separated by 1 mm.
Spinal injections for exclusive labelling of vGLUT2ON neurons
To exclusively label vGLUT2ON neurons in the lumbosacral spinal cord with long-distance projections to the lower thoracic region, we leveraged Boolean logic viral strategies30. Partial laminectomies were made over the L5/L6/S1 spinal segments of vGLUT2–Cre mice. Two sets of bilateral injections of AAVDJ-hSyn-flex-mGFP-2A-synaptophysin-mRuby48 (reference AAV DJ GVVC-AAV-100, Stanford Vector Core Facility) were made over the L5/L6/S1 spinal segments (0.25 µl per injection) at a depth of 0.6 mm below the dorsal surface and separated by 1 mm. Animals were perfused 4 weeks later.
Injection site quantification
To determine the number of transfected neurons within the injection site of the spinal cord, we implemented the spot detection function in Imaris. Following the semi-automatic detection of transfected neurons within representative sections per animal, we quantified the neurons by compiling the exported text file from Imaris.
Perfusions
Animals were perfused at the end of the experiments. Animals were deeply anaesthetized by an intraperitoneal injection of 0.2 ml sodium pentobarbital (50 mg ml−1). Animals were transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. Tissues were removed and post-fixed overnight in 4% PFA before being transferred to PBS or cryoprotected in 30% sucrose in PBS.
Immunohistochemistry
Immunohistochemistry was performed as previously described55,57,58. Perfused post-mortem tissue was cryoprotected in 30% sucrose in PBS for 48 h before being embedded in cryomatrix (Tissue Tek O.C.T, Sakura Finetek Europe B.V.) and freezing. We used two procedures to identify the segment of the spinal cord. First, we identified the dorsal roots in the unsectioned spinal cord. On the basis of the location of the dorsal root entry zones, we prepared well-defined blocks of spinal cord. We then confirmed that the grey matter of the segments possess the expected laminar organization and morphology. Transverse or horizontal sections (30 µm thick) of the spinal cord were cut on a cryostat (Leica), immediately mounted on glass slides and dried or placed in free floating wells containing PBS + 0.03% sodium azide. The sections were incubated with following primary antibody diluted in blocking solution at room temperature overnight: rabbit anti-Fos (1:500), chicken anti-vGLUT1 (1:500), rabbit anti-ChAT (1:100, Sigma-Aldrich) and rabbit anti-Chx10 (now known as Vsx2; 1:500, Synaptic Systems). Fluorescent secondary antibodies were conjugated to Alexa 488 (green), Alexa 405 (blue), Alexa 555 (red) or Alexa 647 (far red; Thermo Fisher Scientific). For the nuclear stain, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; 2 ng ml−1; Molecular Probes). Sections were imaged digitally using a slide scanner (Olympus VS-120 slide scanner) or confocal microscope (LSM880 + Airy fast module with ZEN 2 Black software, Zeiss). Images were digitally processed using ImageJ (ImageJ NIH) software or Imaris (Bitplane, v9.8.2).
Fluorescence in situ hybridization
We performed in situ hybridization of cell-type markers and using RNAscope (Advanced Cell Diagnostics). Lists of putative marker genes were obtained from snRNA-seq data, as described below, and cross-referenced against a list of validated probes designed and provided by Advanced Cell Diagnostics. Probes were obtained for the following genes: ChAT (catalogue no. 408731) and Vsx2 (catalogue no. 438341), Slc17a6 (catalogue no. 319171) and Slc6a5, catalogue no. 409741). We then generated 12-μm cryosections from fixed-frozen spinal cords as previously described59 and performed FISH for each probe according to the manufacturer’s instructions, using the RNAscope HiPlex kit (cat no. 324106). Images were generated using QuPath (v0.4.3).
iDISCO+
Mice underwent a 90-min colorectal distension protocol (30 s inflate then 60 s deflate repeatedly)14 and were perfused9,47 30 min later with 0.1 M PBS followed by 4% PFA (in 0.1 M PBS). Spinal cords were dissected and post-fixed in 4% PFA (in 0.1 M PBS) at 4 °C overnight and placed in 0.1 M PBS containing 0.03% sodium azide. Immunolabelling of the samples was performed by first pretreating with methanol in 5-ml Eppendorf tubes by dehydrating with a methanol–H2O series at 1 h each at room temperature with shaking at 60 rpm: 20%, 40%, 60%, 80% and 100%. This procedure was followed by 1 h washing with 100% methanol before chilling the samples at 4 °C. Samples were then incubated overnight with shaking in 66% dicholoromethane–33% methanol at room temperature. The samples were washed twice in 100% methanol with shaking at room temperature and then bleached in chilled fresh 5% H2O2 in methanol overnight at 4 °C. Samples were rehydrated with a methanol–H2O series: 80%, 60%, 40%, 20% and 0.1 M PBS, each for 1 h at room temperature under shaking. Samples were washed for 1 h × 2 at room temperature in PTx.2 buffer (0.1 M PBS with 0.2% Triton X-100) under shaking. This was followed by an incubation in 5 ml of permeabilization solution (400 ml PTx.2, 11.5 g glycine and 100 ml DMSO for a total stock volume of 500 ml) for 2 days at 37 °C with shaking at 60 rpm. Samples were incubated in 5 ml of blocking solution (42 ml PTx.2, 3 ml of normal donkey serum and 5 ml of DMSO for a total stock volume of 50 ml) for 2 days at 37 °C with shaking. The samples were incubated for 7 days at 37 °C with shaking in primary antibody solution consisting of PTwH (0.1 M PBS, 2 ml Tween-20, 10 mg l−1 heparin, 5% dimethyl sulfoxide and 3% normal donkey serum) and Fos antibody (1:2,000; 226003, Synaptic Systems) for a total volume of 5 ml per sample. Samples were washed in PTwH for 24 h with shaking and incubated for 7 days at 37 °C with shaking in secondary antibody solution consisting of PTwH, 3% normal donkey serum and donkey anti-rabbit Alexa Fluor 647 (1:400, Thermo Fisher Scientific) in a total volume of 5 ml per sample. Samples were washed in PTwH for 24 h with shaking at room temperature. Clearing of the samples was performed by first dehydrating the samples in a methanol–H2O series as follows: 20%, 40%, 60%, 80% and 100% twice each for 1 h with shaking at room temperature followed by a 3-h incubation with shaking in 66% dichloromethane–33% methanol at room temperature. Samples were incubated in 100% dichloromethane 15 min twice with shaking to wash residual methanol. Finally, samples were incubated in 100% dibenzyl ether without shaking for refractive index matching of the solution for at least 24 h before imaging.
Tissue clearing (CLARITY)
We incubated samples in X-CLARITY hydrogel solution (Logos Biosystems) for 24 h at 4 °C with gentle shaking12,55,60. Samples were degassed and polymerized using the X-CLARITY Polymerization System (Logos Biosystems), followed by washes in 0.001 M PBS for 5 min at room temperature. Samples were next placed in the X-CLARITY Tissue Clearing System (Logos Biosystems), set to 1.5 A at 100 rpm at 37 °C for 29 h. Clearing solution was made in-house with 4% sodium dodecyl sulfate, 200 mM boric acid with dH2O, pH adjusted to 8.5. Following this, samples were washed for at least 24 h at room temperature with gentle shaking in 0.1 M PBS solution containing 0.1% Triton X-100 to remove excess sodium dodecyl sulfate. Finally, samples were incubated in 40 g of Histodenz dissolved in 30 ml of 0.02 M PB, pH 7.5, and 0.01% sodium azide (refractive index of 1.465) for at least 24 h at room temperature with gentle shaking before imaging.
3D imaging
We performed imaging of cleared tissue using either a customized mesoSPIM12,61 or a CLARITY-optimized light-sheet microscope (COLM)12. A custom-built sample holder was used to secure the central nervous system in a chamber filled with RIMS. Samples were imaged using either a ×1.25 or ×2.5 objective at the mesoSPIM12,61 or a ×4 or ×10 objective at the COLM12 with one or two light sheets illuminating the sample from both the left and the right sides. The voxel resolution in the x, y and z directions was 5.3 μm × 5.3 μm × 5 μm for the ×1.25 acquisition and 2.6 μm × 2.6 μm × 3 μm for the ×2.5 acquisition. The voxel resolution of the COLM was 1.4 μm × 1.4 μm × 5 μm for the ×4 and 0.59 μm × 0.59 μm × 3 μm for the ×10 acquisition. Images were generated as 16-bit TIFF files and then stitched using Arivis Vision4D (Arivis AG). 3D reconstructions and optical sections of raw images were generated using Imaris (bitplane, v9.8.2) software.
Fos quantifications
For the cleared spinal cords, Fos-positive neurons of cleared samples were quantified using Arivis Vision4D (Arivis)13. After defining a region of interest around the grey matter, each sample was subjected to a custom-made pipeline. We applied morphology, denoising and normalization filters to enhance the signal of bright objects and homogenized the background. Threshold-based segmentation of the Fos signal was applied within predefined 3D regions to quantify the total number of Fos-positive cells. Image analysis parameters were kept constant among all samples. The number of Fos-positive cells and their coordinates enabled us to quantify the neuronal activity segment by segment. For the classic immunohistochemistry, the quantification was done on Imaris (bitplane, v9.8.2) using the spot detection function.
3D reconstruction and quantification
We used the ‘add new surfaces’ tool in Imaris to select the channel of interest, setting the ‘surface detail’ to 0.5 µm for a more detailed surface. The ‘threshold (absolute intensity)’ was adjusted to capture the full shape of the neuron accurately. We applied the ‘number of voxels Img = 1’ filter, selecting the appropriate threshold values to include the reconstructed neuron. After rendering the surface, we made aesthetic adjustments, choosing the ‘transparent 3 – glass’ material and setting the colour to RGB values of (1, 1, 1) to maintain transparency and highlight viral expression. For reconstructing synaptic-like appositions, we created ‘spots’ based on the channel of interest using the spot detection algorithm in Imaris. The ‘estimate xy diameter’ was set between 1.5 µm and 2 µm, with the ‘quality’ filter applied to capture all synaptic-like appositions in the image. The ‘points style/quality’ was set to ‘sphere’ with a ‘radius scale’ of 0.5 µm, using ‘Phong_basic’ as the material for the synapses. To filter synaptic-like appositions to neurons of interest, we used the ‘find spots close to surface’ function with a 1-µm threshold and selected only the ‘spots close to the surfaces’ to display the synaptic-like appositions on neurons of interest. Of note, synapses were primarily counted on neuronal cell bodies as labelling of the different axonodendritic compartments with in vivo immunohistochemistry remains limited.
Axon and synapse quantification
To determine spatial enrichment of axon and synapse density within the grey horn of the spinal cord, we implemented a custom image analysis pipeline that includes preprocessing, registration and combination of histological images from different animals. In brief, we implemented all preprocessing in Fiji, and all registration procedures in R, using the image analysis package ‘imageR’ and the medical image registration package ‘RNiftyReg’. After dynamic registration, all data were summarized and final quantifications were completed using custom R scripts.
Opto-tagging-based neuron-specific recordings and analysis
SCI at T4 and infusion of AAV5-Syn-flex-Chrimson was made in the lower thoracic spinal cord of Vsx2–Cre mice at least 4 weeks before terminal experiments. Mice were anaesthetized with urethane and isofluorane. Two-shank, multi-site electrode arrays (A2x32-6mm-35-200-177, NeuroNexus) were lowered into the spinal cord to a depth of 1,000 µm, with shanks arranged longitudinally at 350 µm from the midline. Signals were recorded with a NeuroNexus Smartbox Pro using a common average reference and while applying 50-Hz notch and 450–5,000-Hz bandpass filters. Stimulation was controlled with a Multi-Channel Systems STG 4004 and MC_Stimulus II software. ChrimsonR-expressing neurons were identified using optogenetic stimulation. Twenty pulse trains of 10-ms pulses were delivered at 10 Hz from a 635-nm laser (LRD-0635-PFR-00100-03, LaserGlow Technologies). Laser light was delivered to the surface of the spinal cord through a fibre optic cable attached to 400 µm, 0.39 NA cannula with a 5-mm tip (Thorlabs). Optical power was set to 2.35 mW at the tip. Electrical stimulation (EES) consisted of 5-ms pulses delivered every 1 Hz. EES was delivered with a micro fork probe (Inomed, 45 mm straight, item no. 522610) positioned along the midline just caudal to the recording array. Spike sorting was performed with SpyKING CIRCUS (v1.0.773). The median-based average electrical stimulation artefacts for each channel were subtracted from the recordings before sorting. Owing to the size and variability of the artefacts, periods containing residual stimulation artefacts were not sorted (–0.5 to +1.5 ms and −0.5 to +1 ms around stimulus onset for EES and laser stimulation onset, respectively). Sorting results were manually curated using Phy (https://github.com/cortex-lab/phy). Single-unit clusters were selected for analysis based on their biphasic waveforms and template amplitudes above 50 µV, as well as strong refractory period dips in their spike autocorrelograms. Similar clusters were merged according to the Phy manual clustering guide. ChrimsonR-expressing, putative SCTHORACIC::Vsx2 neurons were identified based on their low-latency and low-jitter responses to light pulses. Neurons responding to EES or tail pinch were identified by a one-sided Wilcoxon signed-rank test to compare the instantaneous firing rate of units 100 ms before and 100 ms after (EES) or 2 s before and 2 s after (pinch) stimulus onset. For EES, a post-stimulus onset firing rate increase of P < 0.001 was used, whereas for pinch a P = 0.05 was used due to the necessarily lower number of trials and larger calculation window (minimum of 6 trials for pinch and 60 trials for EES).
Statistics, power calculations, group sizes and reproducibility
All data are reported as mean values and individual data points. No statistical methods were used to predetermine sample sizes, but our sample sizes are similar to those reported in previous publications55. Haemodynamic assays were replicated three to five times, depending on the experiment, and averaged per animal. For all photomicrographs of histological tissue, staining experiments were repeated independently with tissue from at least four, and in most cases six, different animals with similar results. Statistics were then performed over the mean of animals. All statistical analysis was performed in R using the base package ‘stats’, with primary implementation through the ‘tidyverse’ and ‘broom’ packages. Tests used included one-tailed or two-tailed paired or independent samples Student’s t-tests, one-way analysis of variance (ANOVA) for neuromorphological evaluations with more than two groups, and one-way or two-way repeated-measures ANOVA for haemodynamic assessments, when data were distributed normally, tested using a Shapiro–Wilk test. Post-hoc Tukey tests were applied when appropriate. For regressions, mixed-model linear regression was used in cases of multiple observations, or else standard linear modelling. In cases where group size was equal to or less than three, null hypothesis testing was not completed. The significance level was set as P < 0.05. Throughout the paper, the boxplots show the median (horizontal line), interquartile range (hinges) and smallest and largest values no more than 1.5 times the interquartile range (whiskers). Exclusions of data are noted in the relevant Methods sections. Unless stated otherwise, experiments were not randomized, and the investigators were not blinded to allocation during experiments and outcome assessment.
scRNA-seq
Single-nucleus dissociation of the mouse lower thoracic and lumbosacral spinal segments was performed according to our established procedures19,20. Following euthanasia by isoflurane inhalation and cervical dislocation, the lumbar spinal cord site was immediately dissected and frozen on dry ice. Spinal cords were doused in 500 µl sucrose buffer (0.32 M sucrose, 10 mM HEPES (pH 8.0), 5 mM CaCl2, 3 mM Mg acetate, 0.1 mM EDTA and 1 mM dithiothreitol) and 0.1% Triton X-100 with the Kontes Dounce tissue grinder. Sucrose buffer (2 ml) was then added and filtered through a 40-µm cell strainer. The lysate was centrifuged at 3,200g for 10 min at 4 °C. The supernatant was then decanted, and 3 ml of sucrose buffer was added to the pellet for 1 min. We homogenized the pellet using an Ultra-Turrax and 12.5 ml of density buffer (1 M sucrose, 10 mM HEPES (pH 8.0), 3 mM Mg acetate and 1 mM dithiothreitol) was added below the nuclei layer. The tube was centrifuged at 3,200g at 4 °C and the supernatant poured off. Nuclei on the bottom half of the tube wall were collected with 100 µl PBS with 0.04% BSA and 0.2 U µl−1 RNase inhibitor. Finally, we resuspended nuclei through a 30-µm strainer and adjusted to 1,000 nuclei per microlitre.
Library preparation
snRNA-seq library preparation was carried out using the 10X Genomics Chromium Single Cell Kit (v3.1). The nuclei suspension was added to the Chromium RT mix to achieve loading numbers of 2,000–5,000. For downstream cDNA synthesis (13 PCR cycles), library preparation and sequencing, the manufacturer’s instructions were followed.
Read alignment
We aligned reads to the most recent Ensembl release (GRCm38.93) using Cell Ranger, and obtained a matrix of unique molecular identifier (UMI) counts. Seurat31 was used to calculate quality control metrics for each cell barcode, including the number of genes detected, number of UMIs and proportion of reads aligned to mitochondrial genes. Low-quality cells were filtered by removing cells expressing less than 200 genes or with more than 5% mitochondrial reads. Genes expressed in less than three cells were likewise removed.
Clustering and integration
Before clustering analysis, we first performed batch-effect correction and data integration across the two different experimental conditions as previously described31. Gene expression data were normalized using regularized negative binomial models62, then integrated across batches using the data integration workflow within Seurat. The normalized and integrated gene expression matrices were then subjected to clustering to identify cell types in the integrated dataset, again using the default Seurat workflow. Cell types were manually annotated on the basis of marker gene expression, guided by previous studies of the mouse spinal cord19,63,64,65. Local and projecting neuronal subpopulations were annotated on the basis of Nfib and Zfhx3 expression, respectively15. Following our projection-specific snRNA-seq experiment in uninjured mice, each subsequent experiment was reintegrated with this dataset before subpopulation annotation. This enabled the identification of the same 28 neuronal subpopulations across the three distinct experiments31.
Cell-type prioritization with Augur
To identify neuronal subpopulations perturbed during natural repair, we implemented our machine-learning method Augur19,21. Augur was run with default parameters for all comparisons. To evaluate the robustness of cell-type prioritizations to the resolution at which neuronal subtypes were defined in the snRNA-seq data, we applied Augur at various clustering resolutions and visualized the resulting cell-type prioritizations both on a hierarchical clustering tree66 of neuron subtypes and as a progression of UMAPs. The key assumption underlying Augur is that cell types undergoing a profound response to a perturbation should become more separable, within the highly multidimensional space of gene expression, than less affected cell types. In brief, Augur withholds a proportion of sample labels, then trains a random forest classifier to predict the condition from which each cell was obtained. The accuracy with which this prediction can be made from single-cell gene expression measurements is then evaluated in cross-validation and quantified using the area under the receiver operating characteristic curve.
Clinical studies design and objectives
All experiments were carried out as part of three clinical safety (primary objective) and preliminary efficacy (secondary objectives) trials: STIMO-HEMO (NCT04994886, CHUV, Lausanne, Switzerland), HEMO (NCT05044923, University of Calgary, Calgary, Canada) and HemON (NCT05111093, CHUV, Lausanne, Switzerland). All three trials and subsequent amendments received approval by the local ethical committees and national competent authorities. All participants signed a written informed consent before their participation, which included consent to complete the autonomic dysreflexia component of the ADFSCI. All participants had the option to indicate consent for the publication of identifiable images or videos. All surgical and experimental procedures were performed at the investigational hospital sites (Neurosurgery Department of the Lausanne University Hospital (CHUV) and the Neurosurgery Department of the Foothills Medical Center (Calgary, Canada). The study involved eligibility and baseline assessments before surgery, the surgical implantation of the respective investigational devices, a post-operative period during which EES protocols were configured, and long-term follow-up periods. To date, a total of ten participants have been participating for more than 6 months in the study. More detailed information about these trials can be found in other publications40.
Study participants
Eleven individuals (five women and six men) who had suffered a traumatic SCI participated overall in the four studies. Demographic data and neurological status, evaluated according to the International Standards for Neurological Classification of Spinal Cord Injury1, can be found in Supplementary Table 3.
Neurosurgical intervention
The participant was put under general anaesthesia and was placed in a prone position. Preoperative surgical planning informed the neurosurgeon about the vertebral entry level and predicted optimal position. On the basis of this knowledge, lateral and anteroposterior fluoroscopy X-rays were performed intraoperatively to guide the location of the laminotomies. A midline skin incision of approximately 5 cm on the back was performed, the fascia opened and the muscles were retracted bilaterally. Excision of the midline ligamentous structures and a laminotomy at the desired entry level enabled the insertion of the paddle array at the spinal thoracic level. For participants of the STIMO-HEMO and HEMO trials, a second skin incision or extended opening caudally was made and a second laminotomy was performed in the lumbar area based on the pre-operative planning to allow for the insertion of the lumbar paddle lead. The paddle lead (or leads; Specify 5-6-5, Medtronic or ARCIM Thoracic Lead, ONWARD Medical N.V) were inserted and placed over the midline of the exposed dura mater and advanced rostrally to the target position guided by repeated fluoroscopies. Electrophysiological recordings were conducted using standard neuromonitoring systems (IOMAX, Cadwell Industries or ISIS Xpress, Inomed Medizintechnik). Single pulses of EES (0.5 Hz) were delivered at increasing amplitude to elicit muscle responses that are recorded from the subdermal (Neuroline Twisted Pair Subdermal, 12 × 0.4 mm, Ambu A/S) or intramuscular (Inomed SDN electrodes, 40 × 0.45 mm, Inomed Medizintechnik) needle electrodes to correct for lateral and rostrocaudal positioning. If the paddles were deviating from a straight midline position, small additional laminotomies were made to remove bony protrusions and guide the paddle to a midline placement. Once the final position was achieved, the leads were anchored to the muscular fascia. In the STIMO-HEMO and HemON trials, the back opening was temporarily closed and the participant was put in lateral decubitus. Subsequently, the back incision was reopened and an abdominal incision of about 5 cm was made per implantable pulse generator (IPG) and a subcutaneous pocket was created. In the HEMO trial, incisions of about 5 cm were made bilaterally in the upper buttocks region and subcutaneous pockets were created. The paddle array cables were then tunneled between the back opening and subcutaneous pockets to be connected to the IPGs (Intellis, Medtronic or ARCIM IPG, ONWARD Medical). The IPGs were implanted in the subcutaneous pockets and all incisions were finally closed.
Stimulation optimization
Spatial mapping was guided by the preclinical mechanisms previously described6 and from previous clinical mappings40, and was conducted in three steps: (1) intra-operative mapping to identify which rows of electrodes target the haemodynamic hotspot, and elicit the largest pressor response in the thoracic spinal cord (T10, T11 and T12), (2) post-operative imaging and spinal reconstructions were used to estimate the electrodes that maximize recruitment of the haemodynamic hotspots, and (3) a single 2-h, post-operative mapping session was done to test each row of electrodes on the lead and pick the three configurations with the largest pressor responses. These configurations were tested in both multipolar and monopolar settings and were validated by personalized simulations to ensure that we were optimally targeting the haemodynamic hotspots. Stimulation frequency was defined empirically at 120 Hz for the spatial mapping6,40. The pulse width was 300 μs. The amplitude was set by incrementally increasing the current per configuration until the systolic pressure increased by 20 mmHg, the diastolic pressure increased by 10 mmHg, or the patient did not report any discomfort such as muscle contractions or sensations such as tingling. These mappings were done in a seated position to mimic relevant, daily life orthostatic challenges.
Clinical haemodynamic monitoring
Beat-to-beat blood pressure and heart rate were obtained via finger plethysmography (Finometer, Finapres Medical Systems). Beat-by-beat blood pressure was calibrated to brachial artery blood pressure collected using an arm cuff embedded and synchronized with the Finometer67,68,69,70. Brachial arterial pressure was sampled at 200 Hz, whereas the systolic, diastolic and mean arterial pressures were extracted from the calibrated arterial pressure at 1 Hz. The heart rate was also sampled at 1 Hz. Raw data and automatically extracted haemodynamic parameters were saved and exported from the Finometer.
Orthostatic challenge with the tilt table test
Participants were transferred to a supine position on a table capable of head-up tilt. We applied restraint straps to secure the patient below the knees, across the thighs and the trunk, with the feet stabilized. Resting supine blood pressure was recorded continuously for approximately 5–10 min to establish baseline values. Then, we tilted the patient upright up to a maximum of 70° while recording haemodynamic values and symptoms of orthostatic tolerance. The time to reach the desired tilt angle from supine was achieved in less than 45 s. Participants were tilted until reaching their tolerance threshold or for a maximum duration of 10 min. They were asked not to talk during the test except to inform and grade symptoms. The participant was asked to report any symptoms every 1–3 min. The participant was asked to rank their symptoms between 1–10, 1 being no symptoms at all, and 10 being feelings of dizziness, lightheadedness71 or nausea37,71. The patient was instructed to notify the research team if they needed to be returned to the supine position.
Post-operative blood pressure data
During a tilt test (see the section ‘Orthostatic challenge with the tilt table test’), changes in blood pressure were recorded without stimulation or in response to different types of stimulation (continuous or closed-loop stimulation) using the Finometer (see the section ‘Clinical haemodynamic monitoring’). Change in blood pressure or heart rate was defined as the difference in the average of a 60-s window before the start of the tilt and a 20-s window at 3 min of the challenge. If the participant could not tolerate at least 3 min of the test due to low blood pressure or other symptoms, an average of a 20-s window before the end of the tilt was used. All measurements in seated position were measured with stimulation on for 3–5 min. Change in blood pressure, or heart rate, was defined as the difference in the average of a 20-s window before the start of EES and the average of a 20-s window at 3 min, before stopping stimulation. All signals were smoothed over a 10-s window for illustration. The same processing was used for post-operative, day 1 quantification. In the present study, we report on blood pressure data on the two study participants implanted with the full ARCIM implantable system.
Off-label investigational system
The investigational system used in the STIMO-HEMO and HEMO clinical trials consisted of a set of CE-marked, FDA-approved medical devices used off-label. Two IPGs (lntellis with AdaptiveStim, Medtronic) are connected to their respective paddle leads (Specify 5-6-5 SureScan MRI, Medtronic), both indicated for chronic pain management. A tablet application with a communicator device (Intellis clinician programmer, Medtronic) was used by the clinical team to wirelessly set up the system and optimize the stimulation parameters. A remote control device and transcutaneous charger device (Patient Programmer and Recharger, Medtronic) were used by the participants to charge the IPGs and wirelessly turn the stimulation on and off during daily life and adapt a subset of stimulation parameters defined by the clinical team.
Purpose-built investigational system for restoring haemodynamic stability
The investigational system used in the HemON was purpose-built for restoring haemodynamic stability. A step-wise approach was followed. All participants were implanted with a purpose-built IPG (ARCIM Thoracic System, ONWARD Medical) that communicates with a purpose-built ecosystem of control devices. The first four participants of the HemON clinical trial received an off-label paddle lead (Specify 5-6-5 SureScan MRI, Medtronic), whereas all other participants were implanted with a novel, purpose-built, paddle lead (ARCIM Thoracic Lead, ONWARD Medical).
Purpose-built IPG and communication ecosystem for restoring haemodynamic stability
The purpose-built ARCIM IPG developed by ONWARD Medical is a novel 16-channel IPG developed to deliver targeted EES. It controls and delivers current-controlled stimulation pulses according to predefined stimulation programmes or through commands received in real time to monopolar or multipolar electrode configurations on 16 channels. The IPG consists of a hermetically sealed, biocompatible can that surrounds the electrical components and a rechargeable battery that enables its function. The IPG is composed of two main components: the header containing the connector block that enables connection with two eight-contact lead connectors as well as two coils for charging and communication, and the can with a rechargeable battery and electronics circuits. The IPG was developed according to all applicable standards for medical device development. Conventional biomedical technologies were used to fabricate the IPG and extensive bench, and in vivo testing was performed to verify its performance. The IPG was implanted subcutaneously at the abdominal level and communicates wirelessly with the ARCIM Hub with near-field magnetic induction. This wearable device was worn on a belt over or in proximity to the IPG location and was responsible for wirelessly charging the IPG’s battery and for programming the IPG with stimulation settings received from several user interfaces. The communication between the hub and IPG provides real-time control of stimulation parameters (as fast as approximately 25 ms between command and stimulation execution), allowing integration with a fast closed-loop neuromodulation system. The ARCIM Hub contains a Bluetooth low-energy chip to enable fast, reliable wireless communication with external programmers such as the ARCIM Clinician Programmer, an Android app designed for clinicians to configure and test the implanted system and personalized stimulation programs. When a stimulation program was deemed safe for personal use, the Clinician Programmer can be used to make this stimulation program available to the patient. The patients, or their caregivers, can control the system through the ARCIM Personal Programmer. This Android watch application allows users to select, start and stop stimulation programs, as well as modulate stimulation amplitudes within predefined safety limits ad hoc. Device errors, paddle lead impedances and daily stimulation utilization were extracted from usage logs across all devices. Furthermore, the Clinician Programmer includes an application programming interface (the ARCIM API) that enables other programming softwares to control the stimulation, for example, for closed-loop control of the stimulation. All devices and softwares are adherent to the applicable standards and their performance was extensively tested. The entire system, including the IPG, received the equivalent of an investigational device exemption from the competent Swiss authorities.
Purpose-built paddle lead
The ARC-IM Thoracic Lead developed by ONWARD Medical is a new 16-electrode paddle lead that is designed for selective recruitment of the dorsal root entry zones of the low-thoracic spinal cord with optimal coverage of the T10–T12 spinal levels. More detailed information about this paddle lead can be found in other publications40.
SCI community survey
Ethical approval was obtained from an independent ethics board (Veritas Independent Review Board) and the Research Ethics Board of Université Laval (principal investigator’s institution). Ethical approval from local research ethics boards was also obtained to recruit from SCI centres across Canada. Individuals with SCI (n = 1,479) across Canada were recruited using a national consumer awareness campaign and provided written informed consent43,44. The survey consisted of a series of variables identified by health-care and service providers, researchers and individuals with SCI, including demographics, secondary health complications, comorbidities, SCI-related needs, health-care utilization, community participation, quality of life and overall health ratings43,44. Participants were asked how often they had experienced symptoms of autonomic dysreflexia in the past 12 months and responses were ranked on a six-point ordinal severity scale ranging from 0 (‘never’) to 5 (‘every day’). Participants were also asked whether they received or sought out treatment concerning these symptoms on a two-point scale (‘yes’ or ‘no’), along with the degree to which it limited activities from 0 (‘never’) to 5 (‘every day’). Participants were also asked whether they had experienced specific problems, such as heart disease, in the past 12 months. Participants’ American Spinal Injury Association Impairment Scale was estimated using responses to questions about lesion level and sensorimotor and/or mobility capabilities. A binary approach was used for the evaluation of outcome variables including the level of injury (cervical SCI versus non-cervical SCI), the severity of injury (complete versus incomplete), the presence of autonomic dysreflexia (yes versus no) and autonomic dysreflexia symptoms (yes versus no). For variables ranked on a six-point ordinal scale, lower scores (0–3) were categorized as ‘no’ and higher scores (4–5) were categorized as ‘yes’.
ADFSCI
The ADFSCI is a 24-item questionnaire divided into four sections: demographics, medication, autonomic dysreflexia and orthostatic hypotension. The autonomic dysreflexia section consists of seven items. Each item used a five-point scale to measure the frequency and severity of symptoms related to autonomic dysreflexia, including headaches, goosebumps, heart palpitations, sweating and anxiety, across different situational contexts. Participants were categorized as experiencing symptoms if the item score was higher than 2 or not experiencing symptoms otherwise. For data collection related to the ADFSCI of individuals not involved in the ongoing clinical studies, Conjoint Health Research Ethics Board approval (REB21-0045) was obtained from the University of Calgary. All participants provided written informed consent before providing their responses.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.