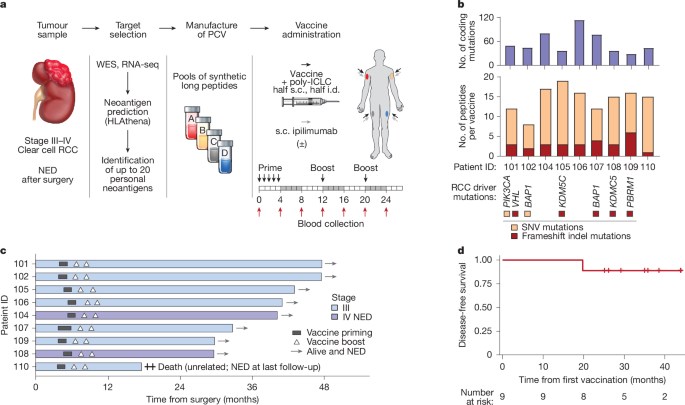
Study design
Patients with presumed high-risk RCC who provided informed consent were screened for eligibility and enrolled in a single-centre, phase I clinical trial approved by the Dana-Farber Harvard Cancer Center Institutional Review Board (NCT02950766). The study was conducted in accordance with the Declaration of Helsinki. Eligibility criteria for final registration included histologically confirmed RCC, American Joint Committee on Cancer (AJCC) stage (eighth edition) stage III (pT3 and/or N1) or fully resected stage IV (pT4 and/or M1 with no evidence of disease). The following additional criteria were applied: Eastern Cooperative Group (ECOG) performance status of 0 or 1; normal organ and bone marrow function; no previous treatment with immunomodulatory agents or RCC-directed cancer vaccine; no previous non-immune systemic therapy within the previous 6 months; no concomitant therapy for cancer or autoimmunity; no history of autoimmune disease or immunodeficiency; and no other non-oncology vaccine within 4 weeks before the first dose of vaccine until 8 weeks after the last dose (with an exception made for vaccination against the SARS-CoV-2 virus). Patients were enrolled in the first cohort (vaccine plus 2.5 mg of subcutaneous ipilimumab at each vaccine site) and the second cohort (vaccine alone) between March 2019 and September 2021. A third cohort of patients treated with vaccine plus 5 mg of subcutaneous ipilimumab at each vaccine site continued enrolment until December 2022 with follow-up ongoing and is not included in this analysis.
The primary objectives of the study were the safety and tolerability of administering the PCV together with locally delivered (subcutaneous) ipilimumab, and the maximum tolerated dose of locally delivered ipilimumab. Secondary objectives were the induction of neoantigen-specific cellular immune responses following vaccination and the proportion of patients alive without recurrence at 2 years after surgery. Each PCV consisted of 4 pools, each containing up to 5 synthetic long peptides targeting neoantigens (300 µg per peptide), admixed with 0.5 mg poly-ICLC (Hiltonol, Oncovir) per pool. Each peptide pool was assigned a non-rotating limb for administration (for example, pool A was always administered in the right thigh). For each administration, each peptide pool was separated into two syringes, and half of the vaccine dose was administered subcutaneously and half intradermally (using separate syringes). For patients who received ipilimumab (patients 101, 102, 104, 105 and 106), it was subcutaneously administered within 1 cm of the vaccine site. The vaccine was administered on days 1, 4, 8, 15 and 22 for vaccine priming and then on weeks 12 and 20 for the booster phase.
Clinical assessments
The safety of treatment was assessed during each vaccine administration and additionally 1 week after the priming phase (day 29), on weeks 8, 13, 16, 21 and 24 and then every 12 weeks during the follow-up phase (up to 2 years). Adverse events were categorized and graded using the NCI Common Terminology Criteria for Adverse Events (CTCAE v.4.0). Dose-limiting toxicity was defined as the following specific toxicities that occurred within 7 weeks of treatment initiation: (1) grade 3–4 toxicity except transient (≤72 h) flu-like symptoms, grade 3 nausea that returns to grade 2 or lower within 48 h or grade 3 rash that resolves to grade 2 or lower within 14 days; (2) grade 3–4 laboratory abnormality that persists for more than 7 days or requires hospitalization or medical intervention; (3) any grade 3–4 toxicity that is considered by the investigator to be dose-limiting; (4) any death related to study treatment. To monitor for disease recurrence, imaging (CT chest, abdomen and pelvis, CT chest and MRI abdomen and pelvis, or PET–CT) was performed at baseline, every 8 weeks up to 24 weeks, then every 12 weeks during the follow-up period (up to 2 years) and then at a frequency decided on by the treating physician (typically every 6 months). Recurrence was assessed using RECIST (v.1.1) guidelines.
Clinical outcomes and safety
Clinical outcomes and safety data are reported until the clinical data cut-off of 7 July 2023. Disease-free survival is reported as the time (in months) of disease recurrence or death from the start of treatment. Analyses and visualizations were generated using the R packages survival (v.3.5.5) and survminer (v.0.4.9). For qualitative comparisons with other recent, randomized clinical trials of immune checkpoint inhibitor as adjuvant therapy for RCC, KEYNOTE-564 (ref. 23) and IMmotion-010 (ref. 16) were selected as the therapeutic settings, and the eligibility criteria of those studies closely matched the current PCV study, specifically adjuvant therapy for high-risk RCC after surgical resection and inclusion of patients with fully resected metastatic disease (termed ‘M1 NED’). The Kaplan–Meier curves for KEYNOTE-564 and IMmotion-010 were reconstructed as previously described58 using DigitizeIt software (v.2.5.9) together with the IPDfromKM package in R (v.0.1.10).
Patient samples
At the time of surgical resection, grossly viable sections of RCC tumour were identified by board-certified, expert trained genitourinary pathologists, and tumour tissue was placed into formalin and into cold DMEM medium (Gibco) on ice. To manufacture the PCV, formalin-fixed tumour tissue was processed and embedded in paraffin blocks at the Dana-Farber Harvard Cancer Center Specialized Histopathology Core, a facility certified by the College of American Pathologists (CAP) and the Clinical Laboratory Improvement Amendments (CLIA). Slides stained with haematoxylin and eosin were prepared from each formalin-fixed paraffin-embedded (FFPE) block to confirm the presence of clear cell RCC. For the two patients with stage IV (metastatic disease), both patients had a single metastatic site in the abdomen, and this was surgically resected at the time of nephrectomy. Separately, FFPE blocks from the primary tumour in the kidney and from the metastatic site were prepared and both were used for the design of the vaccine. The block with the highest tumour purity and lowest necrosis was selected for preparation of FFPE thick sections (scrolls), which was transported to the Broad Institute for isolation of DNA and RNA for sequencing studies. All material used for production of the clinical vaccine followed a strict chain of custody, with multiple standard checkpoints in place to ensure the correct identity of the specimen.
For research (non-clinical) analyses, fresh tumour tissue and adjacent non-malignant kidney tissue were placed in separate tubes containing DMEM on ice. Sections of tumour and normal tissue were enzymatically and mechanically dissociated as previously described38. Fresh tissue was minced with scalpels to 1–2 mm3 fragments and incubated in digestion medium containing 0.11 U ml–1 collagenase D (Roche), 0.56 U ml–1 dispase (Stemcell), 50 U ml–1 DNase I (NEB), 5 mM CaCl2 and HBSS (Life Technologies) at 37 °C for 10 min with agitation. Single-cell suspensions of tumour or normal kidney were then cryopreserved in 10% DMSO plus 90% FBS for downstream use.
Peripheral blood samples were obtained before or at the time of surgery for HLA typing and for germline DNA sequencing. Once eligibility was confirmed, clinical HLA class I and II typing was performed by the BWH Tissue Typing Laboratory. For immune monitoring of PBMCs, leukaphereses were performed before vaccination (within 10 days) and at week 16 following vaccine initiation. Peripheral blood samples (K2-EDTA tubes) were also collected at weeks 3, 8, 12, 20 and 24 following vaccine initiation (with additional specific samples collected at the discretion of the investigator). PBMCs were isolated by Ficoll (GE Healthcare) density centrifugation and then cryopreserved for downstream analyses. The plasma layer following density centrifugation was frozen at –80 °C for downstream analyses of circulating soluble protein.
For analyses of skin-infiltrating immune cells, two 5-mm punch biopsy samples were obtained from non-sun-exposed skin (right proximal thigh for the pretreatment and post-vaccine priming samples, and right volar forearm for the cutaneous DTH assessment). Skin samples were minced using scalpels, incubated in digestion enzymes (Miltenyi Whole Skin Dissociation kit, using enzyme P and enzymes A + D), incubated at 37 °C for 2 h with agitation and further dissociated using a Miltenyi gentleMACS (program h_skin_01). The single-cell suspension was then enriched for CD45+ immune cells through immunomagnetic purification (Miltenyi anti-CD45 microbeads) and immediately used for downstream analyses.
Generation of neoantigen-targeting PCVs
The creation of each PCV involved WES of tumour tissue and peripheral blood, RNA-seq of tumour, somatic mutation calling, identification of target neoantigens and then synthesis, pooling and preparation of the vaccine product. For the two patients with metastatic disease, tumour material from both the primary tumour and the single resected metastatic site underwent sequencing and analysis for vaccine design.
For WES of tumour and normal blood, FFPE tumour scrolls and a peripheral blood sample were transported to the Broad Institute Clinical Research Sequencing Platform (CRSP), a CLIA licensed (22D2055652) and CAP accredited (8707596) laboratory for the standard somatic WES test. DNA derived from FFPE samples was extracted using a Qiagen DNA FFPE QIAamp kit per the recommended protocol. Deparaffinization solution (DPS) from the kit was added to the samples and incubated on a heat block at 56 °C per the manufacturer’s recommendations. Following the DPS treatment, buffer ATL and proteinase K from the kit were added to the samples and incubated on a heat block overnight at 56 °C and then at 90 °C in a heat block for 1 h the following day. The lysate was then transferred to a new tube, and RNAse A, buffer AL and ethanol were added. The lysate was transferred to a QIAmp spin column, and wash buffers included in the kit were added. The sample was finally eluted with the provided ATE buffer and stored at 4 °C for downstream processing. Following extraction of total DNA from blood and tumour tissue using these standardized procedures, library construction was performed. Library construction was performed as previously described59, with the following modifications (as previously reported60): the initial genomic DNA input into shearing was reduced from 3 µg to 20–250 ng in 50 µl of solution. For adapter ligation, Illumina paired-end adapters were replaced with palindromic forked adapters (Integrated DNA Technologies) with unique dual-indexed molecular barcode sequences to facilitate downstream pooling. For end repair and A-tailing, adapter ligation and library enrichment PCR, Kapa HyperPrep reagents were used in a 96-reaction kit format. For the post-enrichment SPRI clean-up, the library concentration was maximized by reducing the elution volume to 30 µl to maximize library concentration. Libraries were pooled (up to 96 samples), and hybridization and capture were performed using an Illumina’s Nextera Exome kit. The following modifications were made from the manufacturer’s protocol: (1) all libraries in a library construction plate were pooled before hybridization; (2) a skirted PCR plate was used to facilitate automation (Agilent Bravo liquid handling system) instead of the Midi plate from Illumina’s Nextera Exome kit. After post-capture enrichment, library pool quantification was performed using qPCR (automated assay on the Agilent Bravo) probes specific to the ends of the adapters (KAPA Biosystems). Libraries were normalized to 2 nM and then cluster amplification was performed according to the manufacturer’s protocol (Illumina) using exclusion amplification chemistry and flowcells. The flowcells were analysed using RTA (v.2.7.3 or later). Each pool of whole exome libraries was sequenced on paired 76 cycle runs on Illumina HiSeq or NovaSeq instruments with two 8-cycle index reads across the number of lanes (or equivalent) needed to meet coverage for all libraries in the pool. The anticipated read depth for most samples was >150× mean target coverage.
RNA-seq was performed to confirm the expression of putative neoantigens. For all patients, a separated portion of tumour tissue (FFPE scroll) was sent to the non-CLIA/CAP Broad Institute Genomics Platform. RNA derived from FFPE samples was extracted using a Qiagen AllPrep DNA/RNA FFPE kit per the recommended protocol. DPS from the kit was added to the samples and incubated at 56 °C per the manufacturer’s recommendations. Following the DPS treatment, the samples were placed on a heat block at 37 °C to evaporate any residual buffer. Once all residual buffer was removed, buffer PKD and proteinase K from the provided kit were added and the samples incubated at 56 °C. The samples were then centrifuged to collect the DNA-containing pellet and RNA-containing supernatant. The RNA supernatant was transferred to a new tube and incubated on a heat block at 80 °C. Buffer RLT from the kit and ethanol was added to the lysate and was applied to a RNeasy MinElute spin column. A series of wash buffers and ethanol were applied to the bound RNA for purification. The sample was finally eluted with the provided RNase-free water and denatured at 65 °C and flash chilled on wet ice. The purified RNA was then stored at –80 °C for downstream processing. Following RNA extraction using these standardized procedures, transcriptome capture RNA-seq was performed using an optimized protocol for RNA from FFPE tissues (Broad Institute). Total RNA was quantified using a Quant-iT RiboGreen RNA Assay kit and normalized to 5 ng µl–1. Following plating, 2 µl of ERCC controls (using a 1:1,000 dilution) were spiked into each sample. RNA quality was measured using a Caliper LabChip GX system, which was used to calculate a RIN-equivalent RNA quality score. The per cent of RNA fragments greater than 200 nucleotides were also quantified into a DV200 score. Using a TruSeq RNA Access Library Prep kit (Illumina), a stranded cDNA library was prepared from isolated RNA, which was then hybridized to a set of DNA oligonucleotide probes to enrich the library for mRNA transcript fragments. Flowcell cluster amplification and sequencing were performed according to the manufacturer’s protocols using Illumina HiSeq or NovaSeq S2 instruments. Each run was a 76 bp paired-end with an eight-base index barcode read. Data were analysed using the Broad Picard Pipeline, which includes demultiplexing and data aggregation. Alignment was completed using the STAR alignment algorithm against human reference hg19. Transcriptome capture covers the RefSeq and GENCODE (v.12) databases to >98%.
When available, additional RNA-seq of purified tumour cells (that is, without other cells from the microenvironment) was performed. CA9-positive tumour cells were immunomagnetically purified (using Miltenyi anti-CA9 PE antibody and anti-PE microbeads) from freshly dissociated tumour and cultured for 48–72 h in OptiMEM GlutaMax medium (Gibco) containing 5% FBS, 1 mM sodium pyruvate, 100 U ml–1 penicillin and streptomycin, 50 µg ml–1 gentamicin, 5 µg ml–1 insulin and 5 ng ml–1 EGF. RNA was extracted using a Qiagen RNeasy kit, and RNA-seq of this low-input RNA was performed using a modified version of the Smart-seq2 protocol (using the NEBNext Single Cell/Low Input cDNA Synthesis and Amplification module) as previously described3.
Somatic mutations in WES data were detected using an implementation of the Cancer Genome Analysis WES Characterization pipeline (as previously implemented10; hereafter, referred to as the CGA pipeline) in a cloud-based analysis platform, Terra (https://terra.bio/). We used v.0.2 of the CGA pipeline. As quality-control steps, the CGA pipeline ran deTiN61 and ContEst62 for estimating tumour-in-normal and cross-patient contaminations, respectively. The pipeline applies multiple artefact filters such as the read-realignment filter by BLAT63 and the read orientation bias filters followed by SNV and indel calling with MuTect64 and Strelka65. All somatic alterations were annotated using Oncotator66. Tumour ploidy and purity were determined using ABSOLUTE67, and this was used to assign clonality (cancer cell fraction) for each somatic mutation. RNA-seq data were quantified as previously described10. In brief, RNA-seq data were aligned to the human reference genome (hg19) and transcriptome (GENCODE v.19) using STAR (v.2.6.1)68, and expression was quantified (transcripts per million) using RSEM (v.1.3.1)69. All coding mutations were visualized using the Integrated Genomics Viewer (IGV v.2)70. Each somatic mutation was categorized as high quality or green (variant read support in WES and RNA-seq), yellow (high quality in WES but not identified in RNA-seq) or red (likely artefact). Green-categorized mutations were prioritized for downstream analyses, although yellow-categorized mutations were also included.
To predict neoantigens, the detected coding mutations were converted into peptide sequences, and the translated neoantigens (that is, peptides with missense mutations and indels) were sliced into 8, 9, 10 or 11 amino-acid lengths for HLA class I binding prediction. Antigen prediction was performed using HLAthena (v.0)71 and NetMHCpan (v.4)72 (eluted ligand rank). Each potential antigen was ranked as follows: (1) predicted strong binder (rank < 0.5) by both prediction models; (2) predicted strong binder by HLAthena only; (3) predicted strong binder by NetMHCpan only; (4) predicted weak binder (rank < 2) by both prediction models; (5) predicted weak binder by HLAthena only; (6) predicted weak binder by NetMHCpan only; (7) not a predicted binder.
For each patient, an epitope selection board (ESB) was convened, which consisted of investigators with expertise in RCC, genomics, immunobiology, cancer immunotherapies and peptide chemistry. The ESB selected up to 20 short epitopes based on mutation quality, antigen rank, cancer driver status (favouring driver over passenger mutations), expression (in bulk tumour or in the purified tumour RNA; favouring high expression), clonality (favouring clonal over subclonal mutations) and whether the somatic alteration was a frameshift indel capable of producing a novel open reading frame. The list of driver genes for this cohort was created on the basis of known cancer genes and significantly mutated genes in the Cancer Genome Atlas–clear cell kidney cancer cohort (TCGA–KIRC)30 and PanCancer lists from the TumorPortal73 (http://www.tumorportal.org/). Genes with detected somatic mutations were intersected with the driver gene list, and the nonsynonymous mutations in the driver genes were marked as driver mutations. For the two patients with metastatic disease, we prioritized neoantigens that were present in both the primary and the metastatic tumour. A small number of neoantigens were also selected that were present in only the primary tumour or in only the metastatic tumour. For the selected short epitopes, the ESB designed synthetic long peptides (15–33 amino acids in length) containing the peptide sequence flanking the short epitope, with a length and sequence intended to maximize synthesizability and solubility.
Good manufacturing practice peptides were synthesized using standard solid-phase synthetic peptide chemistry and purified by reverse-phase high-performance liquid chromatography (RP-HPLC; Neon Therapeutics for patients 101 and 102, Almac Sciences for the other patients). Each individual peptide was analysed using various qualified assays to assess appearance (visual), purity (RP-HPLC), identity (by mass spectrometry) and quantity. Peptides were combined into one of four pools containing up to five peptides (designed to maximize the diversity of predicted HLA class I binding allele and excluding peptides that did not demonstrate clear solutions; the assigned pool for each peptide is listed in Supplementary Table 2). Each peptide pool was sterile-filtered and underwent additional testing for sterility and the presence of endotoxin. On the day of administration. Each pool was thawed, mixed with poly-ICLC (Hiltonol, Oncovir) and separated into syringes for subcutaneous and intradermal administration, as described above.
For cutaneous DTH testing, 0.25 ml of each vaccine pool (containing 100 µg of each peptide and without poly-ICLC or ipilimumab) was combined (1 ml total volume), and 100 µl of this mixture of all vaccine peptides was intradermally administered in the right forearm 7–14 days after the week 12 vaccine priming dose. At 48–72 h following injection, the DTH site was assessed for erythema and induration, and biopsies were performed (two 5-mm punch biopsies) at the injection site and samples were processed in the same manner as other skin biospecimens, as described above.
Assessment of vaccine immunogenicity by IFNγ ELISpot
PBMCs containing circulating T cells were assessed for immune reactivity to vaccine peptide pools immediately after thawing and rest (ex vivo), to individual vaccine peptides following in vitro stimulation with pools of peptides, and to autologous tumour cells following in vitro stimulation with individual peptides or peptide pools. All T cell culture and ELISpot methods (including plate development and analysis) were performed as previously described74,75.
For assessing the dynamic ex vivo immunogenicity of vaccine peptide pools over the course of vaccination, PBMCs containing T cells spanning week 0 (before first vaccination) until week 24 (after vaccine initiation) were screened against pooled neoantigen vaccine peptides by ex vivo IFNγ ELISpot assays. Opaque ELISpot plates with 0.45 mm hydrophobic high protein binding immobilon-p membranes (Millipore) were wet with 35% ethyl alcohol. Wells were washed with distilled water three times. Plates were coated with anti-IFNγ antibody at 4 °C (1-DK1 Mabtech) and were then washed with PBS before stimulation. Cryopreserved PBMCs from the nine patients were thawed in complete DMEM medium supplemented with 10% human serum and rested overnight. The PBMCs were then plated at 2 × 105 cells per well and stimulated with 10 µg peptide pool. DMSO and/or 10 µg HIV peptides were used as negative controls. CMV, EBV and influenza-derived (CEF) peptide pools (Mabtech) and PHA (Gibco) were used as positive controls. Each stimulation was set up in triplicate and incubated for 18–24 h at 37 °C. Plates were developed using manufacturer’s protocols. For each timepoint, responses were background-subtracted (that is, the median of the negative controls were subtracted). Responses were defined as positive if they showed >55 spot-forming units per 106 PBMCs and at least threefold increase over baseline.
Neoantigen vaccine peptide pools were deconvoluted to identify immunogenic peptides by in vitro IFNγ ELISpot assays. To generate vaccine-pool-specific T cells, week 0 and week 16 PBMCs were plated in 24-well plates at 5 × 106 to 1 × 107 cells per well and stimulated with 10 µg ml–1 vaccine peptide pools in DMEM medium supplemented with 10% human serum and 20 ng ml–1 of IL-7 (PeproTech). On day 3, the T cells were cultured with DMEM medium supplemented with 10% human serum and 20 U ml–1 of IL-2 (PeproTech). In vitro IFNγ ELISpot assays were performed 10–14 days after stimulation, after resting the T cells in cytokine-free medium overnight. For these assays, 3 × 104 cells were plated against 10 µg individual vaccine peptide (negative controls, DMSO and 10 µg of HIV; positive controls, CEF peptides and PHA). No reactivity was detected in week 0 PBMCs. For week 16 PBMCs, positive peptide responses were identified as those that were significantly increased compared with the negative control (DMSO; using a two-sided t-test and a significance threshold of 0.05, without adjustment for multiple comparisons) and that had at least threefold median increase in the number of IFNγ spot-forming units over the negative control.
T cells that were reactive to individual peptides and/or peptide pools were further assessed for reactivity to autologous tumour cells. Dissociated tumours (either all dissociated cells or tumour cells immunomagnetically enriched using anti-CA9 antibodies and Miltenyi microbeads) were either used directly or pre-stimulated with 250–2,000 units of IFNγ (PeproTech) 2–3 days before ELISpot assays were performed (to increase antigen presentation). For quantifying the tumour reactivity of T cells stimulated with vaccine peptide pools, 3 × 104 T cells were plated against 1 × 104 autologous (that is, patient-specific) unstimulated and IFNγ-stimulated (IFNγ+) tumour cells. For individual immunogenic vaccine peptides, antigen-specific T cell lines derived from week 0 (control) and week 16 PBMCs were generated and cultured as described above. Antigen-specific T cell lines were then tested 10–14 days after stimulation using in vitro IFNγ ELISpot assays. To confirm immunogenicity and tumour reactivity, 3 × 104 T cells were plated with DMSO (negative control), against 10 µg of individual immunizing long peptides or against 1 × 104 tumour cells. Positive peptide (or peptide pool) responses were identified as those that were significantly increased compared with the negative control (DMSO; using a two-sided t-test and a significance threshold of 0.05, without adjustment for multiple comparisons) and that had at least threefold median increase in the number of IFNγ spot-forming units over the negative control.
Assessment of T cell immunophenotype and cytokine production by flow cytometry
PBMCs were thawed and rested overnight at 37 °C in RPMI (Life Technologies) containing 10% human serum (Sigma-Aldrich), 1% penicillin and streptomycin (Life Technologies) and 20 ng ml–1 of IL-7 (PeproTech). The following day, PBMCs were stimulated with vaccine peptide pools (2 µg ml–1 per individual peptide) in complete R10 medium (RPMI, 10% FBS and 1% penicillin and streptomycin). After 1 h of co-culture at 37 °C, transport inhibitors GolgiStop and GolgiPlug were added. Following 5 h of incubation at 37 °C, samples were washed with 1× PBS and stained with live/dead dye in 1× PBS for 20 min at 4 °C. Samples were subsequently washed with staining buffer (1× PBS, 10% FBS and 1% penicillin and streptomycin). Fc receptors were blocked for 15 min at 4 °C with Human TruStain FcX in staining buffer. The following surface-staining antibodies were then added: CD4 (clone L200), CD8 (clone RPA-T8), CD45RO (clone UCHL1) and PD-1 (clone EH12.2H7). Following 30 min of incubation at 4 °C, samples were washed twice before fixation and permeabilization with CytoFix/CytoPerm for 15 min at 4 °C. Samples were then washed twice and resuspended in 1× Perm/Wash buffer to conduct intracellular staining with IFNγ (clone B27), TNF (clone Mab11), IL-2 (clone MQ1-17H12) and CD3 (clone UCHT1). Following 30 min of incubation at 4 °C, samples were washed twice with Perm/Wash, once with staining buffer and then resuspended in CytoFix fixation buffer until acquisition. All flow cytometry reagents were purchased from BD Biosciences or BioLegend. Data were acquired on a BD LSR Fortessa with FACSDiva and analysed using FlowJo (v.10.8.1). Background responses, as determined by DMSO control, were subtracted from peptide pools to determine the frequency of neoantigen-specific cytokine responses. Samples had to have at least 50 IFNγ+ T cells to be included in the analysis. Furthermore, the frequency of IFNγ+ cells had to be at least 1.5-fold higher than in the negative control, HIV gag, to be included. When CD4+ and CD8+ T cells did not meet these stringency thresholds, the results are reported as ‘n.d.’ for ‘not detectable’, and not include in the summary statistics. When these criteria were not met for CD8+ T cells specifically (but were for CD4+ T cells), the proportion of CD8+ T cells was set to zero for that sample. Finally, the HIV gag negative control was not available for assessments of samples from patient 110, and so although the individual results are reported in Extended Data Fig. 5, they are not included in the summary Fig. 2b.
Measurement of circulating soluble proteins
The levels of circulating cytokines, chemokines and other soluble plasma proteins before and during vaccination were quantified using an Olink Target 96 Immuno-Oncology assay. Stored plasma from week 0 (before vaccination), week 3 (during vaccine priming) and week 20 (before the second booster dose and 8 weeks after the previous vaccine administration) was transported to the Human Immune Monitoring Center at the Icahn School of Medicine at Mount Sinai for Olink analysis. All values are reported as normalized protein expression (in arbitrary units). For Supplementary Table 3, for each cytokine, the median value across all patients is reported. Statistical testing was performed using a two-sided paired Wilcoxon test, comparing pretreatment (week 0) with either week 3 or week 20 timepoints (excluding patient 104, who did not have pretreatment measurements available).
scRNA-seq and scTCR-seq
scRNA-seq and single-cell TCR-seq (scTCR-seq) were performed as previously described38. All skin samples were processed immediately (that is, fresh) following dissociation, whereas tumour and normal kidney samples were thawed (after previously being cryopreserved). Dead cells were immunomagnetically depleted using a Dead Cell Removal kit (Miltenyi), washed and resuspended in PBS with 0.04% BSA at a concentration of 1,000 cells per µl. Approximately 17,000 cells were loaded onto a 10x Genomics Chromium instrument (10x Genomics). Libraries were prepared using a Chromium single cell 5′ kit v.2 (for gene expression) and a Chromium single-cell human TCR amplification kit (for scTCR-seq). Following library construction, quality control and quantification, libraries were pooled and sequenced on an Illumina NovaSeq S4. The 10x Cell Ranger pipeline (v.6.1.2) was used for data demultiplexing, data alignment and count quantification and TCR clonotype assembly.
Analysis of scRNA-seq and scTCR-seq data from vaccine-site skin
scRNA-seq data were imported and read using a custom function built on the Seurat (v.4.3.0) pipeline76. The function discriminates between raw and filtered Cell Ranger outputs, reads the 10x data, creates the Seurat object and calculates the mitochondrial content. For raw input Cell Ranger data, a filtering step that removes cells with nFeature_RNA < 5 was also performed, before returning the preprocessed Seurat object. Quality control was performed following the standard Seurat pipeline. Cells with fewer than 200 genes, fewer than 500 unique molecular identifiers (UMIs) and more than 20% mitochondrial content were excluded. Doublets were identified and removed using three different methods: doubletFinder77 (v.2.0.3), scDblFinder78 (v.1.12.0) and nFeature_RNA > 2,500. Any cells identified as doublets by any of these three methods were excluded from further analyses.
Following preprocessing, the skin data were harmonized using the package Harmony (v.0.1.1)79 with a maximum iteration setting of 20. Thereafter, the skin data were clustered using Seurat’s FindClusters function with default options, and the optimal clustering resolution was determined using the clustree function from the package clustree (v.0.5.0)80. The immune subset was then identified and subsetted on the basis of expression of the immune marker PTPRC (which encodes CD45). The immune subset was further clustered using the methodology outlined above. Despite conservative doublet filtering, one cluster (cluster 9) was identified as consisting of doublet cells and removed from further analyses. Cluster 11, identified as keratinocytes and not immune cells, was also excluded.
Differential gene expression analysis was performed using the FindAllMarkers function from Seurat with standard options. The purpose of this analysis was to identify differentially expressed genes characterizing each immune cluster. After clustering the immune subset and performing differential gene expression analysis, myeloid and T cell populations were identified on the basis of canonical markers, including CD33 and CD3E expression, respectively. After isolating myeloid and T cell compartments, each population was further clustered to identify T cell and myeloid subpopulations. Differential gene expression analysis was performed for each myeloid and T cell subcluster as described above.
To identify changes in populations during vaccination (that is, from week 0 before vaccination to week 4 following vaccine priming) and any impact of subcutaneous administration of ipilimumab (that is, comparing week 4 skin in patients who received vaccine alone versus those who received vaccine plus ipilimumab), we performed a population abundance analysis. Specifically, we focused on each subcluster in the myeloid and T cell compartments. The proportions for population abundance were calculated by dividing the number of cells in each subpopulation by the total number of the parent population (either T cells or myeloid cells) per sample. Significance of change in frequency over time (week 0 versus week 4) was assessed using a two-tailed, paired Wilcoxon test, whereas differences across treatment was determined by a two-tailed, unpaired Wilcoxon test. Two patients (105 and 107) were excluded from the paired analysis owing to the lack of samples at week 0 (too few immune cells for library construction and sequencing). Boxplots of population abundances are shown with the median, the 25th and 75th percentiles (box) and 1.5× the interquartile range (whiskers).
To determine any phenotypic changes that may occur in a specific population following vaccination, we performed differential gene expression analysis for each cluster (week 0 versus week 4) using the function FindMarkers from Seurat. Heatmaps were generated using the R package pheatmap (v.1.0.12).
For TCR diversity and clonotype analysis, we used the R package VDJdive (v.1.3.5)81. This package was used to assess the clonal repertoire diversity and abundance in the TCR sequences. Clonotype labels were assigned using the clonoStats function, with the following parameters: method = “EM”, type = “TCR”, assignment = TRUE, group = “sample”. TCR diversity was calculated using the calculateDiversity function, using the normentropy method. This measure provides a normalized entropy score that quantifies the diversity of TCR clonotypes in each sample, based on a matrix of clonotype abundances, with higher scores indicating greater clonal diversity. The significance of the differences in the number of unique clonotypes and in TCR diversity between week 0 and week 4 skin-infiltrating T cells was assessed using a two-tailed paired Wilcoxon test.
Analysis of scRNA-seq and scTCR-seq data from tumour and normal kidney
scRNA-seq data preprocessing, harmonization and clustering and identification of T cell populations were performed as described above for the skin analysis. One cluster (cluster 15) was identified as a mitochondrial contaminant and removed from further analyses. Patient-specific TCR clonotypes were identified using TCRα and TCRβ chains and CDR3 region amino-acid sequences. Barcodes with two TCRα and two TCRβ, or with three or more TCRα or TCRβ chains were removed from further analyses using a custom R function built on the combine TCR function in the R package scRepertoire (v.1.8.0)82. Then, TCR clonotypes were integrated into scRNA-seq data using cell barcodes and were ordered by patient-specific clonal expansion. Overall, 74,865 T cells in tumour samples had paired single-cell RNA and TCR clonotype data.
We inferred the tumour or viral specificity of infiltrating T cells using previously defined gene expression signatures28. Module scores were calculated for each tumour T cell with normalized RNA data using the Seurat function AddModuleScore. Scores were calculated for both the tumour-specific (TS) T cell gene signature and a virus-specific (VS) T cell gene signature. The gene sets were composed of upregulated genes with log2 fold change above 1 from experimentally validated TS or VS CD8+ tumour-infiltrating lymphocytes, respectively. The module scores were used to quantify the enrichment of genes from a given gene signature in each cell. The gene signature score of each TCR clonotype was then determined by averaging the TS and VS signature score for each T cell in their clonotype. The density distribution of individual T cells and TCR clonotypes across TS and VS was assessed and, based on the dominating VS of normal T cells compared with tumour T cells and the near mutually exclusive distribution of TS and VS signature scores across TCR clonotypes, score thresholds were defined to infer the specificities of individual T cells and TCR clonotypes. Putative TS T cells and TCR clonotypes had TS scores greater than 0 and VS scores less than 0. Putative VS T cells and TCR clonotypes had VS scores greater than 0 and TS scores less than 0. Overall, the signature score threshold inferred 2,581 TS and 1,328 VS TCR clonotypes and comprised 37,007 and 5,650 inferred TS and VS T cells, respectively. Furthermore, for each TS and VS TCR clonotype, the percentage of cells with the same specificity as their clonotype was calculated. An average of 76.6% and 71% of cells were inferred to be TS across TS clonotypes and VS across VS clonotypes, respectively, according to the signature score thresholds described above. TS and VS clonotypes were filtered to retain those composed of 75% or more of TS cells or VS cells, respectively. Following this additional filtering, there were 1,635 TS and 270 VS TCR clonotypes. Finally, TS TCR clonotypes were further filtered by those clonotypes with expansion in both normal and tumour tissue to exclude non-tumour-specific clonotypes. TCR singletons and doubletons were excluded from further analyses. Final inclusion parameters consisted of TS TCR clonotypes seen in tumours at a count between three and five and not seen in normal cells. For clonotypes seen in tumour above five counts, the count in normal cells must have been at least five cells fewer.
TCR-seq of peripheral blood T cells
Bulk sequencing of TCRβ chains from peripheral T cells (PBMC samples) was performed as previously described28,83. Raw data were processed using MiXCR (v.4.4.2)84 and aligned using the built-in reference annotation (repseqio.v3.0.1) using the ‘generic-amplicon-with-umi’ preset, with the following additional parameters (–export-productive-clones-only –rna–tag-pattern ‘^(R1:*)\(UMI:N85)(R2:*)’ -Massemble.consensusAssemblerParameters=null -Massemble.cloneAssemblerParameters.addReadsCountOnClustering=true–floating-left-alignment-boundary –floating-right-alignment-boundary C). From the list of observed clonotypes for each technical replicate, we ran the exportClonesOverlap function with the following parameters (-tagCounts -vGene -jGene –criteria “CDR3|AA|V|J”) to cluster all clonotypes with identical CDR3 amino acid sequence, variable (V) genes and joining (J) genes to generate consensus clonotypes for each biological sample. To further aggregate clonotypes, we merged TRBV genes (TRBV6-2, TRBV6-3, TRBV6-5 and TRBV6-6; TRBV12-3 and TRBV12-4) that are indistinguishable based on the locations of the RNase H-dependent PCR-enabled TCR-seq (rhTCR-seq) primers, as previously described83. We then removed all UMIs supported by only one read and removed clones with no remaining UMIs. Clonotypes in a given sample with identical J genes and CDR3 amino acid sequences were then collapsed into the clonotype with the highest UMI count. Last, a report file was generated with aggregated UMI counts for each clonotype and the total number of unique clonotypes identified. These post-processing steps were written in Python and available at GitHub (https://github.com/Wu-Lab-DFCI-Harvard/bulkrhTCR_Script).
Four replicates were sequenced for each timepoint. In brief, CD3+ T cells were immunomagnetically isolated from thawed PBMCs samples (using anti-CD3 microbeads; Miltenyi), RNA was isolated (Qiagen) and rhTCR-seq was performed. This method uses RNase H-dependent PCR to enhance the specificity of TCR amplification and incorporates UMIs to each cDNA molecule to improve the accuracy of clonotype frequency estimations. Sequencing was performed on an Illumina MiSeq or a NextSeq 1000, and then MiXCR84 was used to assemble TCRβ clonotypes.
Inference of tumour specificity, viral specificity and vaccine specificity in peripheral blood T cells
The proportion of TS and VS T cells in the periphery was determined by matching clonotypes from the scTCR-seq analysis and the bulk TCR analysis. For each patient, the scTCR clonotypes were linked to bulk TCR clonotypes by matching the TCRβ V gene, J gene and the CDR3β amino acid sequence. The β-chains were separated for scTCR clonotypes with two identified β-chains. Owing to the high granularity of scTCR, multiple scTCR clonotypes were discovered to have the same β-chain identity but different α-chain identities. For each patient, an average (across all patients) of 19.9 TS clonotypes and 3.7 VS clonotypes had shared β-chains but unique α-chains. However, no β-chains were shared between TS and VS scTCR clonotypes. This matching process identified the inferred TS and VS TCR clonotypes in the peripheral T cells. For each patient and each timepoint, the frequencies of all inferred TS TCRs separately all inferred VS TCRs were reported for each individual patient at each timepoint. The arithmetic mean (and s.e.m.) and frequency of inferred TS and VS TCRs was graphed across all timepoints.
To infer the peripheral TCR clonotypes with vaccine-specificity, we identified TCR clonotypes that were either absent or just at the lower limit of detection in the pre-vaccination (week 0) sample, and then expanded by at least 10-fold in all of the subsequent timepoints (and having at least 3 UMI sequencing reads at ≥2 timepoints). For TCR clonotypes that were undetectable, their value was set to the nearest order of magnitude less than the smallest observed TCR frequency (that is, lower limit of detection) for that patient (10−4 for all patients). To increase stringency, we further required that these clonotypes be present in the scTCR analysis of the cutaneous DTH assessment (at week 13). The percentage frequency of all vaccine-inferred clonotypes were reported for each patient at each timepoint, and the arithmetic mean (and s.e.m.) of the percentage frequency of inferred vaccine-specific TCRs was graphed across all timepoints. For visualization in Fig. 4d and Extended Data Fig. 8b,c, later timepoints (collected past the 24-week protocol-mandated timepoints), TCR measurements were binned and displayed at the middle timepoint of the interval (weeks 50–74 were grouped and displayed at week 62, weeks 75–99 were grouped and displayed at week 87, weeks 100–124 were grouped and displayed at week 112, weeks 125–149 were grouped and displayed at week 137, and weeks 150–174 were grouped and displayed at week 162).
To infer whether each expanded clonotype consisted of CD4 or CD8 T cells, we traced clonotypes to single cells using the TCRβ chain and integrated two complementary approaches for clonotype annotation. First, we used the paired scRNA-seq and TCR data from the skin (DTH assessment at week 13) to classify the T cell clonotypes as CD4 or CD8. Specifically, we used our per cluster annotation (as defined in Fig. 3 and Extended Data Fig. 9a,b). Next, we used a per cell annotation through reference mapping to a human CD4 (https://doi.org/10.6084/m9.figshare.21981536.v1) and CD8 atlas (https://doi.org/10.6084/m9.figshare.23608308.v1) with the tool ProjectTILs (v.3.3.1)86. The majority of clonotypes (53 out of 98) had concordant phenotype annotations by both methods, defined by >75% of their cells being classified as CD4 or CD8. An additional 25 clonotypes were annotated as CD4 or CD8 by only one of the methods given an unresolved annotation by the second method. For 11 clonotypes that had discordant CD4 versus CD8 annotation by the 2 methods, the expression of CD4 and CD8A were manually evaluated and cells were annotated on the basis of the unique detection of one of these genes, which resulted in an additional 3 clones annotated as CD4 or CD8. We additionally excluded one cell as its matching TCR clonotype comprised two β-chains, thus we could not unambiguously match the clonotypes observed in the (unpaired) bulk TCR data. The remaining cells remained as unresolved (that is, could not confidently annotated as CD4 or CD8).
Statistics and reproducibility
Given the limited primary patient material, experiments were typically performed a single time (when appropriate, technical replicates were performed during that experiment). In limited cases (specifically for IFNγ ELISpot assays), experiments may have been repeated if there was a failure of positive or negative controls and there was sufficient primary patient material to repeat the experiment.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.