Animals
All experimental procedures were carried out in accordance with National Institutes of Health (NIH) guidelines and approved by the Institute Animal Care and Use Committee and the Institute Biosafety Committee at the California Institute of Technology (Caltech). Mice were housed in ventilated micro-isolator cages in a temperature- and humidity-controlled environment under a reverse 12-h light/dark cycle. Food and water were provided ad libitum. Mouse cages were changed weekly. Esr1flp knock-in mice (JAX no. 036028), Slc32a1(Vgat)cre knock-in mice (Jax no. 028862), Slc17a6(Vglut2)cre knock-in mice (Jax no. 028863), Ai6 (Jax no. 007906), were backcrossed into the C57BL/6N background (more than N10) and bred at Caltech. BALB/c female mice, ovariectomized and intact, were used as intruder mice and purchased from Jackson Laboratory. Pth2cre knock-in mice were bred at Caltech and purchased from GemPharmatech (stock no. T054004). Heterozygous Vglut2cre, Pth2cre or double heterozygotes Esr1flp, Vgatcre, Pth2cre Ai6 mice were used for cell-specific targeting experiments and were genotyped by PCR analysis using genomic DNA from tail or ear tissue. Adult mice were 8–12 weeks old at the time of viral injection. Animals were randomly assigned to different experimental conditions. Group sample sizes were chosen on the basis of previous studies.
Viruses
AAVDJ-hSyn-Con/Fon-hChR2(H134R)-eYFP and AAV2-syn-Flex-jGCaMP8s were packaged at the HHMI Janelia Research Campus virus facility. AAVRetro-EF1a-DIO-hChR2(H134R)-eYFP, AAVDJ-hSyn-Con/Fon-eYFP, AAV2-hSyn-DIO-hM4D-mCherry, AAV2-hSyn-DIO-mCherry, AAV8-EF1a-Nuc-flox(mCherry)-EGFP and AAV1-hSyn1-SIO-stGtACR2-FusionRed were purchased from Addgene. AAV9-EF1a-DO-hChR2(H134R)-mCherry, AAV8-DIO-TC66T-2A-eGFP-2A-oG, G-Deleted Rabies-mCherry and EnvA G-Deleted Rabies-mCherry were purchased from Salk Institute. AAVDJ-hSyn-DIO-ChR2(H134R)-eYFP was purchased from the UNC Vector Core. ‘Con/Fon’ indicates Cre-ON/Flp-ON virus; ‘DO’ indicates Cre-OFF virus. All virus titres were 1012 or more genomic copies per millilitre.
Stereotaxic surgery
Surgeries were performed on adult mice aged 8–12 weeks. Mice were anaesthetized with 1–3.0% isoflurane and placed into a stereotaxic alignment system (David Kopf Instruments). A small craniotomy hole was drilled with a dental drill. Virus (0.1–0.3 μl; titre, 1012 particles per ml) was then injected into the target area using a pulled glass capillary (World Precision Instruments) and a pressure injector (Micro4 controller, World Precision Instruments), at a flow rate of 20 nl min−1. The capillary was then slowly retracted at least 5 min after infusion. Stereotaxic injection coordinates were based on the Paxinos and Franklin atlas (MPOA, bregma −0.1, midline ±0.5, dorsal surface −4.7; SPFp bregma −3.1, midline ±1.5, dorsal surface −3.7; SpV bregma −6.5, midline ±1.8 and dorsal surface −5.2). For optogenetic and fibre photometry experiments, fibre optic cannulas (optogenetics: diameter 200 μm, numerical aperture 0.22; fibre photometry: diameter 400 μm, numerical aperture 0.5; Thorlabs) were subsequently placed 300–500 μm above the virus injection sites and fixed on the skull with dental cement (Parkell). Mice were allowed to recover for at least 2 weeks before behavioural testing. Half of the mice in each cage were randomly assigned to either treatment or control groups. All control mice were treated with the same experimental procedures, but a control virus was injected instead.
For acute electrophysiological recordings combined with optogenetics, viral injections were performed and an implanted fibre optic cannula and a steel headplate was fixed to the skull with dental cement. One day before electrophysiological recordings, animals were briefly anaesthetized and a 32 AWG chlorinated silver wire (A-M system) with a presoldered gold pin was implanted through a small hole and cemented to the skull to provide chronic grounding. One or more craniotomies (less than 1 mm in diameter) were drilled and then covered with a drop of silicone oil (30,000 cSt, Aldrich) followed by a silicone sealant (KwikCast, World Precision Instruments) until the experiment was performed. Animals were single housed following surgery and allowed to recover before recordings.
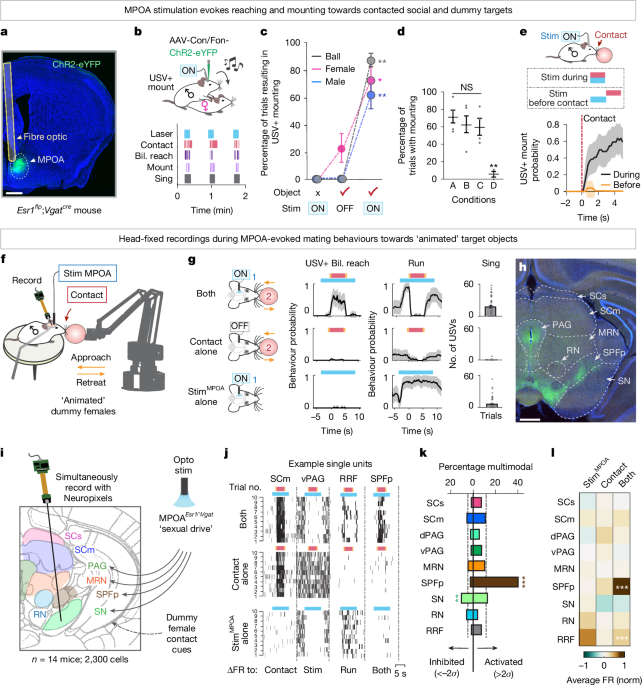
For MPOAEsr1∩Vgat optogenetic recording experiments, viruses encoding intersectional61,62 (Cre and Flp)-dependent channelrhodopsin (Con/Fon-ChR2-eYFP) was injected unilaterally into the MPOA, ipsilateral to the recording site in Esr1flp;Vgatcre mice. For MPOAEsr1∩Vgat and SpVVgat− dual optogenetic experiments, Con/Fon-ChR2-eYFP was injected into the ipsilateral MPOA and Cre-OFF ChR2 (DO-ChR2-mCherry) was injected into the contralateral SpV in Esr1flp;Vgatcre mice. For SpVVglut2−>SPFp optogenetic recording experiments, a retrogradely labelling Cre-dependent virus (AAVRetro-DIO-ChR2-eYFP) was injected into the SPFp and the fibre optic was implanted over the contralateral SpV in Vglut2cre mice. For SPFpPth2 optogenetic experiments, Cre-dependent ChR2 (activation) or stGtACR2 (inactivation) was injected bilaterally into the SPFp in Pth2cre mice. For SPFpPth2 chemogenetic experiments, Cre-dependent hM4Di was injected bilaterally into the SPFp in Pth2cre mice. For SPFpPth2 fibre photometry experiments, Cre-dependent GCaMP8s63 was injected unilaterally into the SPFp in Pth2cre mice.
For monosynaptic rabies tracing, viruses encoding Cre-dependent mutated TVA (TC66T) and rabies G glycoprotein was injected into the SPFp of Vgatcre, Vglut2cre or Pth2cre animals35. Two weeks later, EnvA G-Deleted Rabies-mCherry was injected into the same location in the SPFp36. Mice were housed in a biosafety room for 4–6 days to allow the rabies virus to infect and express mCherry in presynaptic cells before the brains were harvested.
Electrophysiological recordings
Mice were head-fixed, the silicone sealant was removed and physiological saline was applied to the skull to cover the craniotomy. Electrophysiological recordings were made using Neuropixels 1.0 probes, recording from the 384 most distal channels64. The reference and the ground contacts on the Neuropixels probes were permanently soldered together. Recordings were made using an external reference configuration achieved by connecting the probe reference to the chronically implanted silver wire on the skull. To allow post hoc tracking of probe trajectories, the electrode shank was coated with DiI (Invitrogen). The Neuropixels probe was attached to an aluminium dovetail adaptor screwed to an aluminium rod and lowered with a micromanipulator (Sensapex) at roughly 2 μm s−1 until reaching the target depth. Data acquisition began 20 min after reaching the final depth. Neural signals were acquired at 30 kHz using Open Ephys software65. For optogenetic stimulation, a fibre optic cannula was connected to a blue 470-nm laser (Shanghai Laser and Optics Century). Before data acquisition, the laser was turned on to check for a photoelectric artefact in the neural recording. Animals in which the laser power that reliably induce behaviours was too high and, therefore, produced a photoelectric artefact were not included. After each recording experiment, probes were slowly retracted and immersed in 1% Tergazyme solution to remove tissue and silicone oil residues.
For the initial large-scale screen, we recorded from a total of 2,300 single units from 9 nine brain regions across 14 mice. This included 110 units in SCs, 442 units in SCm, 148 units in dorsal periaqueductal grey, 356 units in numerical aperture ventral periaqueductal grey, 465 units in midbrain reticular nucleus, 268 units from SPFp, 140 units from the substantia nigra, 164 units from the red nucleus and 207 single units from the retrorubral field. Including this screen, we recorded from a total of 837 single units from the SPFp across 18 mice (11 with MPOA and/or SpV stimulation, seven without stimulation).
Optogenetic stimulation
Mice with fibre optic implants were connected to an optic fibre (200-μm diameter, numerical aperture 0.22; Doric Lenses) and allowed to habituate before behavioural testing. The optic fibre was connected to a 470-nm laser to deliver blue light (Shanghai Laser and Optics Century). Before behavioural testing, the light intensity achieved at the tip of the optic fibre was estimated by connecting an equivalent optic fibre to the patch cable and measuring the light intensity at the tip of the fibre using a power meter. Laser power was controlled by turning an analogue knob on the laser power supply. The laser was either triggered manually when animals were engaged in a behaviour of interest or automatically in a series using a Pulse Pal (Open Ephys). For optogenetic stimulation in freely behaving and head-fixed animals, mice were given trains of photostimulation (10-ms pulse, 20 Hz for 10 s). Sham stimulation (laser off) was interleaved as an internal control. For freely moving SPFpPth2 optogenetic experiments, animals were given a 5-min baseline interaction period. Then, 2 sets of 12 photostimulation and 12 interleaved sham stimulation trials were delivered every 30 s using a Pulse Pal. This ensured randomized stimulation periods throughout the interaction. Frequency titration experiments involved series of 1, 5, 10 and 20 Hz pulse trains delivered by the experimenter on approach of the female.
Chemogenetic inhibition
Mice were injected with hM4Di-mCherry66 or mCherry (control)-expressing AAVs in the MPOA of Pth2cre mice. Males were sexually naive and single housed 1 day before behavioural testing. Behavioural tests were performed with at least 1 day in between tests. Mice were intraperitoneally injected with CNO (Enzo; 5.0 mg kg−1) dissolved in saline 40 min before behavioural testing. All animals received CNO or saline on the same day with the experimenter blind to the treatment group.
Fibre photometry
To measure bulk florescence, mice with fibre optic implants were connected to an optical fibre (400-μm diameter, 0.5 numerical aperture; Thorlabs) to both deliver excitation of light and collect emitted florescence. We used 470-nm light-emitting diodes (LEDs) (M470F3, Thorlabs, filtered with 470–10-nm bandpass filters FB470-10, Thorlabs) for fluorophore excitation and 405-nm LEDs for isosbestic control (M405FP1, Thorlabs, filtered with 410–10-nm bandpass filters FB410-10, Thorlabs). Each excitation wavelength (470 and 405 nm) was sinusoidally modulated at a distinct carrier frequency (208 and 333 Hz, respectively) that is demodulated to recover the original calcium sensor response (that is, lock-in amplification)67. This modulation step minimizes contamination of the calcium signal by changes in overall ambient light and low-frequency noise. The emission signal from the 470-nm excitation was normalized to the emission signal from the isosbestic excitation (405 nm), to control for motion artefacts, photobleaching and levels of GCaMP8 expression. LEDs were coupled to a 425-nm longpass dichroic mirror (Thorlabs, DMLP425R) by means of fibre optic patch cables (diameter 400 μm, numerical aperture 0.5; Thorlabs). Emitted light was collected through the patch cable, coupled to a 490-nm longpass dichroic mirror (DMLP490R, Thorlabs), filtered (FF01-542/27-25, Semrock), collimated through a focusing lens (F671SMA-405, Thorlabs) and detected by the photodetectors (model no. 2151, Newport). Recordings were acquired using Synapse software (Tucker Davis Technologies).
Mice were habituated to the optic fibre cable before behavioural testing. For behaviour testing, mice were presented with an inanimate object (ball), a hormonally primed female under a pencil cup and then free access to a hormonally primed female. All data analyses were performed in MATLAB. Behavioural videos, audio and fibre photometry data were time-locked. Fn was calculated using normalized (405 nm) fluorescence signals from 470-nm excitation. Fn(t) = 100 × (F470(t) − F405fit(t)/F405fit(t)). For z-scored data, traces were z-score normalized before averaging.
Histology
Verification of virus expression, implant placement and Neuropixels probes (DiI) were performed on all mice. Mice lacking correct viral expression or probe targeting were excluded from analysis. Mice were transcardially perfused with saline, followed by 4% paraformaldehyde in 1× PBS. Brains were collected and postfixed overnight, then cryoprotected in 30% sucrose. Brains were embedded in optimal cutting temperature mounting media, frozen on dry ice and sectioned coronally at 40–100 μm on a cryostat (Leico Biosystems). Sections were immunolabeled and counterstained with 4,6-diamidino-2-phenylindole (0.5 μg ml−1) washed and mounted onto slides. Primary antibodies were rabbit-anti-DsRed (1:1,000, Takara Bio 632496) and goat anti-Fos (1:500, Santa Cruz, sc52-g). Secondary antibodies were Alexa Fluor 594 donkey anti-rabbit (1:1,000, Invitrogen, A-21207) and Alexa Fluor 647 donkey anti-goat (1:1,000, Invitrogen, A-21447). FISH (RNAScope, ACD Bio) was performed with probes targeting Pth2 (1052361), mCherry (431201), GFP (409011), SLC32a1 (VGAT, 319191) and SLC17a6 (VGLUT2, 319171) following the manufacturer’s protocol. Sections were imaged with an epifluorescent (Olympus VS120) or a confocal microscope (Leica).
Behavioural assays
Head-fixed behaviours during acute recordings
Object presentation was controlled by a robotic arm (ufactory, uArm) that allowed for the selection of xyz coordinates, speed and duration of presentation. Top and side infrared cameras (Basler, acquired at 10 Hz) were used to record behaviours and triggered in Python. Audio recordings were collected at a 250-kHz sampling rate using an Avisoft-UltraSoundGate116H kit with a condenser ultrasound microphone CM16/CMPA (Avisoft-Bioacoustics). Laser stimulation was triggered by a Pulse Pal (Open Ephys) that was time-locked to the robotic arm controlled in Python. All video, audio, laser and robotic arm TTL signals were concurrently acquired on a NIDAQ (National Instruments) and later aligned to neural recordings in MATLAB.
Object trial bouts involved presentations of an object (ping pong ball, toy mouse and so on) that approached a mouse, maintained position for 5 s and then retracted. Laser trial bouts involved 10 s of 10-ms pulses at 20 Hz. Multimodal trial bouts involved either laser on first and after a 2-s delay the object was presented or the reverse. Interstimulus intervals were at least 5 s within a bout and several minutes between trial bouts. Trial bouts (object, laser and multimodal) were randomly interleaved. For presentation of conspecifics, BALB/c females or males were briefly anaesthetized and presented with the anogenital region towards the face of the recorded mouse. For presentation of objects with female odours, urine from the anogenital region of a BALB/c female mouse was swabbed onto a ball to be immediately presented using the robotic arm. The location of object contact was varied to touch different regions of the body, lasting 5 s for each trial. For object features, the same object was presented that varied in temperature, textures or robot-controlled pressure.
Behavioural monitoring
All behavioural experiments were performed during the animals’ dark cycle. Mice were habituated in the room for 10–20 min before testing. Behavioural tests were performed in conventional mouse housing cage (home cage or new cage) using the previously described behaviour recording set up68. For optogenetic experiments, most behaviour tests were performed under white light. All other tests were performed under red light. Both top and front cameras (forward-looking infrared, Grasshopper) acquired video at 30 Hz using StreamPix7 (Norpix). Audio recordings were collected at a 250-kHz sampling rate using an Avisoft-UltraSoundGate116H kit with a condenser ultrasound microphone CM16/CMPA (Avisoft-Bioacoustics) that was positioned 45 cm above the cage. Video and audio recordings were synchronized through a signal generated by StreamPix7.
Hormone priming
To enhance the sexual receptivity of female mice, hormone primed ovariectomized BALB/c female mice were used as stimulus animals in some experiments (chemogenetic, Fos, fibre photometry). Females were injected subcutaneous with 10 μg of β-estradiol-3-benzoate (E8515, Sigma-Aldrich) in sesame oil (S3547, Sigma-Aldrich) at 48 h, and 500 μg progesterone (P0130, Sigma-Aldrich) at 4–6 h before behavioural testing3,69. Females were tested before the assay for receptivity with a stud male mouse until up to three mounting attempts occurred, and any that demonstrated rejection behaviours were not used.
Mating assay
Male mice were single housed before testing. On the test day, males were acclimated in his home cage under the recording set up. A sexually experienced BALB/c female mouse was then introduced into the male’s cage either for free interaction or under a pencil cup (controls). For MPOA optogenetic experiments, animals were given 20 min to interact with a female or an object (ball) with randomly interleaved laser and sham stimulation. For sensory testing conditions, male mice received optogenetic stimulation with an object under white light, infrared light, following whisker trimming and whiskerless with topical lidocaine applied to the face in that order. For c-Fos induction, animals were allowed to interact until ejaculation and then perfused 1 h after ejaculation or 1 h after time matched exposures to a female under a pencil cup (controls). For chemogenetic and fibre photometry experiments, animals were given 1 h to interact with a female. At the end of hM4Di experiments, animals were either given saline or CNO and allowed to interact with a female for 1 h to induce c-Fos. To reduce the baseline mounting for SPFpPth2 optogenetic experiments, sexually naive males (never ejaculated) were used and paired with either sexually naive C57BL/6N or ovariectomized unprimed BALB/c females. None of these interactions proceeded into intromission as the females rejected most mount and intromission attempts. This allowed for shorter but more frequent re-initiation of reach and mount attempts.
Data analysis
Behaviour annotations
Behaviour videos (collected at 30 Hz) of mating interactions were first processed using a custom automated behaviour classifier system (the Mouse Action Recognition System, MARS) to generate frame-by-frame annotations of mounting and investigation behaviour70. Classifier annotation output, videos and spectrograms of recorded audio were then loaded into a custom, MATLAB-based behaviour annotation interface and classifier annotations were manually corrected by trained individuals blind to the experimental design. For head-fixed behaviours, videos were manually annotated. Final annotations include investigate (sniff), approach, chase, bilateral reach, mount, intromission and ejaculation. Bilateral reaching was defined as moments reaching for a conspecific or object when both of the male mouse’s forelimbs were off the ground. Mounting was defined as moments when both forelimbs were off the ground and the chest of the animal was on top of the conspecific or object. Intromission was defined as moments when the animal intromits, characterized by slow thrusting. Ejaculation was defined as the moment just preceding the male mouse falling over. Mount, reach or intromission bouts were considered terminated when all four limbs of the male mouse were on the ground, either when the male disengaged or when the female ran away. Thus, reaching and mounts during the pursuit of a female results in a series of shorter bouts whenever the distance between them exceeds the length of the animal’s forelimbs.
USV detection
USV audio files were saved as in the 16-bit WAV format and later analysed with DeepSqueak v.3.0, a deep learning-based software for detection and analysis of USVs71. The built-in mouse call detecting network was used to identify USV syllables and then corrected by manual inspection. Detection files were then exported and analysed in MATLAB. The number of USVs as a function of time was calculated and aligned with video and neural recordings.
Motion tracking
Videos of head-fixed mice acquired at 10 frames per second, were cropped to regions of interest (ROIs). ROIs corresponded to the region just below the mouse’s body to track limb motion during running (run ROI) and the region just in front of the chest to track limb motion during reaching (reach ROI). ROIs were converted to motion energy (change in pixel intensity from frame to frame) and the top 500 principal components were extracted for each ROI by singular value decomposition using FaceMap72. The first principal component of the motion data was used to correlate to the mean smoothed neural activity trace to obtain an r value.
Preprocessing and spike sorting
Neural signals from electrophysiological recordings were preprocessed by subtracting the median calculated within each group of 24 channels from the data to eliminate common-mode noise. The median subtracted data were spike sorted using Kilosort2.5 that in addition to the group median subtraction applied a high-pass filter (150 Hz), followed by whitening in blocks of 32 channels73. The cluster automatically labelled by Kilosort algorithm as ‘good’ were in turn manually curated by hand and further analysed with Phy2.
Firing rates
Spikes for each neuron were binned at 100-ms resolution and binned counts were divided by the bin width. For latency analysis, spikes were instead binned at 10-ms resolution. For some analyses, the rates were z-scored across the duration of the session per neuron. Baselines were the average number of spikes for a given 4 s before stimulus onset. Activated and inhibited cells were defined as responses at least 2σ from baseline. For clustering analysis, the z-scored trial average rates (20 s trials, 200 bins) were concatenated by neuron and k-means clustering was performed.
Selectivity index and choice probability
The selectivity index for each neuron was computed based on the average firing rate to two conditions (contralateral versus ipsilateral touch). The index was based on previous studies and is defined as: (responsecontra − responseipsi)/(responsecontra + responseipsi). Units with a high selectivity index (greater than 0.3) were considered contralateral selective. Units with low selectivity index (less than −0.3) were considered ipsilateral selective. These units were then selected for further analysis.
The choice probability of each neuron was computed as in previous work. Choice probability estimates the accuracy with which two conditions can be distinguished given the activity of each neuron. The choice probability of a given neuron for a pair of conditions (contralateral versus ipsilateral) is found by constructing a histogram of the activity of that cell (F(t)) under each of a selected pair of conditions and plotting the histograms against each other to generate a receiver operating characteristic curve. The area under this receiver operating characteristic curve is then computed by integration to generate the choice probability value for each unit with respect to each of the two conditions. This choice probability value is bounded from 0 to 1, with a choice probability of 0.5 indicating that the activity of the neuron cannot distinguish between the two conditions. Values greater than 0.7 or less than 0.3 were selected for further analyses.
Integration index
The integration index was computed for each neuron and was based on the average firing rate in a 5-s window for each condition (object only, MPOA stimulation only and multimodal). The integration index was computed based on previous studies: (responseMulti − responseadditive)/(responseadditive) × 100, where responseadditive = (responseMonly + responseOonly). Units with an integration index above zero were considered superadditive integration cells and selected for further analysis.
Anatomical registration of electrode tracks
Electrode tracks were traced using DiI in coronal sections and registered to the Allen Mouse Brain Common Coordinate Framework74. Alignment was performed using SHARCQ to morph the atlas based on any asymmetries in the coronal slice and register locations of fluorescence75. Electrode locations were mapped using the Allen Mouse Brain Common Coordinate Framework and then cross-checked with the Franklin–Paxinos atlas and manual inspection. All single unit data used in the study were tagged with a corresponding location to the brain ROI. Units outside these anatomical boundaries were not included.
Cell counts
A brain map was overlain on the digital image to identify the appropriate regions using landmark structures, ventricles and optic tract as reference guides. An experimenter blind to condition analysed the images in ImageJ. For cell counts, the number of labelled cells were analysing using the automated ImageJ cell counter.
Statistical analysis
Data were processed and analysed using Python, MATLAB and GraphPad (GraphPad PRISM v.9). The sample sizes were chosen on the basis of common practice in animal behaviour experiments. No statistical methods were used to predetermine sample size. Data were first tested for normality using a Shapiro–Wilk test and tested for homogeneity of variance using a Levene’s test. If data met normality and homogeneity of variance assumptions, parametric tests were used (for example, Student’s t-test, one-way or repeated-measures analysis of variance with Tukey’s multiple comparison). If not, non-parametric tests were used (for example, Mann–Whitney U-test or Kruskal–Wallis with Dunn’s multiple comparison test). Paired tests were used to compare within-group repeated-measures data (for example, Wilcoxon signed rank test and a Friedman test with Dunn’s multiple comparison). All statistical tests were two-sided. Significance levels are indicated as follows: *P < 0.05; **P < 0.01 and ***P < 0.001. For all representative images, similar results were obtained in at least three independent experiments.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.