Animals
All animal procedures followed ethical guidelines outlined in the NIH Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Institutional Animal Care and Use Committee at Harvard Medical School. Mice were maintained under constant environmental conditions (23 ± 1 °C, 46 ± 5% relative humidity) with food and water provided ad libitum under a 12 h–12 h light–dark cycle. All of the studies used adult male and female mice (aged 6–24 weeks) in comparable numbers from mixed genetic backgrounds. Tmie-, Gpr68- and Otop1-knockout mice were gifts from J. Holt, A. Patapoutian and E. Liman. All of the other mice were purchased from Jackson Laboratory, made in the laboratory and then deposited at Jackson Laboratory, or received as gifts and later deposited in the Jackson Laboratory: C57BL/6J (00664), Vglut2-ires-cre (16963), Npy2r-ires-cre (29285), Piezo2-ires-cre (27719), Phox2b-cre (16223), P2ry1-ires-cre (29284), Pvalb-t2a-cre (12358), Glp1r-ires-cre (29283), Oxtr-t2a-cre (31303), lsl-ChR2 (12569), lsl-DTR (07900), lsl-SALSA (31968), lsl-tdTomato (07914), loxP-Piezo2 (27720), loxP-Piezo1 (29213) and Snap25-GCamp6s (25111).
Physiological measurements
Arterial blood pressure was measured in anaesthetized mice (1.5–2% isoflurane in oxygen). The carotid artery or femoral artery was cannulated through a custom-built fluid catheter (Braintree Scientific RPT015, MRE033, MRE065) attached to a pressure transducer (BioPac, TSD104A; Sensor, RX104A) amplified by the Biopac DA100C system. Right atrial pressure was measured by inserting a catheter through the external jugular vein to access the atrium. Electrocardiograms were performed using needle electrodes placed subcutaneously on the right forepaw and left hindpaw, amplified using the Biopac ECG100C system. Breathing was measured using a pressure transducer (Harvard Apparatus, Differential Pressure Transducers MPX) within the isoflurane delivery device, amplified by the Biopac DA100C system. For some experiments, the jugular or femoral veins were cannulated for blood withdrawal or saline (0.9% NaCl) infusion manually or with a syringe pump (NE-1000, New Era Pump Systems). Data were acquired using the Biopac MP160 system with the Acknowledge software.
Tilt-table test
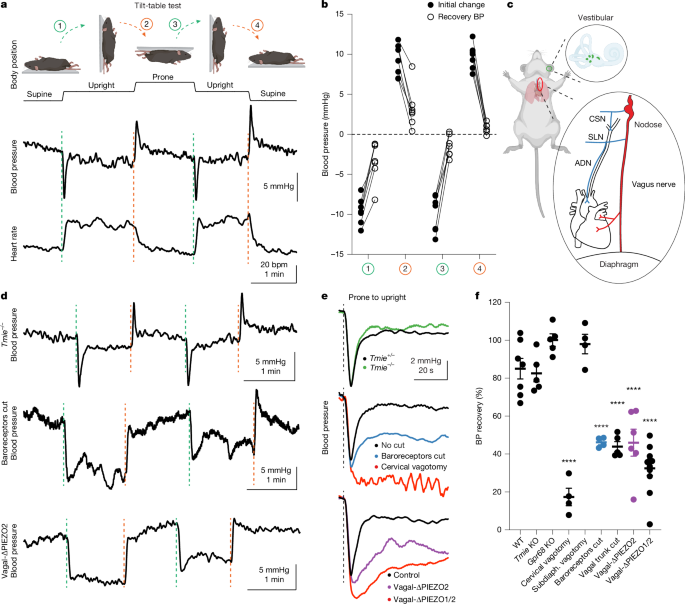
Anaesthetized mice (1.5–2% isoflurane in oxygen) were immobilized on a surgical platform with adhesive, and prepped for measurements of blood pressure, heart rate and breathing. The isoflurane concentration was then reduced to 1.2–1.5% until the breathing rate stabilized at around 60 breaths per minute. The blood pressure transducer was kept level with the catheter site to avoid a hydrostatic effect during rotation. The surgical platform with the mouse was magnetically secured to a tilt table, which was connected to an accelerometer for movement recording. The mouse was manually tilted to different positions, remaining in each position for at least 1 min while the physiological parameters were recorded (Biopac).
Haemorrhage model
Mice were anaesthetized (1.5–2% isoflurane in oxygen) and placed onto a heating pad to maintain the body temperature above 35°C. Heparin (200 U kg−1 in PBS) was administered through the right external jugular vein or tail vein and isoflurane was lowered (1.2–1.5%) until the breathing rate stabilized at around 60 breaths per minute. After 10 min, the tail was transected (2 cm from the tip) with a scalpel to induce bleeding. Blood was collected in an Eppendorf tube and weighed at 30 min and at the survival end point. Survival was continuously assessed by detectable breathing. Animals of different genotypes were used in a random order to which the experimenter was blinded for analyses of survival and heart rate.
Neuron ablation
Vagal sensory neurons were ablated as previously described by serial injection (10–20 injections of 10 nl) of DT (Sigma-Aldrich, D0564) solution (5–20 μg ml−1 DT, 0.05% Fast Green FCF Dye with PBS) into surgically exposed nodose/jugular/petrosal (NJP) superganglia with a Nanoject III injector (Drummond). Control mice were injected with PBS lacking DT. Mice were allowed to recover for at least 3 weeks after injection, and nodose ganglia were collected and immunostained for DTR after experiments to determine the extent of ablation.
AAV injection
The AAVs AAV-flex-tdTomato (Addgene, 28306-AAV9), AAV-eGFP (Addgene, 105542-AAV9) and AAV-flex-eGFP (Addgene, 51502-AAV9) were purchased. For the experiments in Fig. 2a,b and Extended Data Figs. 4 and 5, surgically exposed NJP superganglia were serially injected (20 injections of 10 nl) with AAV-flex-tdTomato (AAV titre > 1 × 1013 vg per ml, 0.05% Fast Green FCF Dye, Sigma-Aldrich). For Fig. 2d, NJP superganglia were injected (30 injections of 10 nl) with a 2:1 injection solution of AAV-flex-tdTomato (AAV titre > 1 × 1013 vg per ml and 0.05% Fast Green FCF Dye, Sigma-Aldrich) and AAV-eGFP (AAV titre > 1 × 1013 vg per ml and 0.05% Fast Green FCF Dye, Sigma-Aldrich). For Extended Data Fig. 4, the left NJP superganglion was injected with AAV-flex-eGFP (AAV titre > 1 × 1013 vg per ml, 0.05% Fast Green FCF Dye, Sigma-Aldrich) and the right NJP superganglion was injected with AAV-flex-tdTomato (AAV titre > 1 × 1013 vg per ml, 0.05% Fast Green FCF Dye, Sigma-Aldrich). All injections were performed using the Nanoject III injector (Drummond).
Whole-tissue clearing and immunostaining
Mice were anaesthetized (avertin, 1 ml, 12.5 mg ml−1) and perfused with PBS (20 ml) and paraformaldehyde (10 ml, 4%, PBS) administered through the left ventricle. The heart and attached vasculature were removed by dissection, fixed (4% PFA, PBS, overnight, 4 °C) and washed twice in PBS with 0.05% NaN3 (2 h at room temperature). For Fig. 2a, whole-heart clearing with Adipo-Clear was performed as previously described with modifications49. In brief, refractive index matching was conducted using ethyl cinnamate (Sigma-Aldrich 112372). After clearing, the samples were degassed in a vacuum desiccator for several hours to remove bubbles trapped in the heart chambers. The samples were imaged using light-sheet microscopy (UltraMicroscope II by LaVison, ImSpector v.7.1.4). For Fig. 2b,d and Extended Data Figs. 4 and 5, thick section clearing was performed on intact left atria, intact right atria, intact aorta and thick ventricle slices (1 mm, Zivic Instruments, Mouse Heart Slicer Matrix, HSMA001-1). Thick tissue samples were cleared using the CUBIC protocol as previously described50,51. Tissues were incubated (2 h, 37 °C) in blocking solution (2% normal donkey serum (Jackson ImmunoResearch, 017-000-121), 0.1% Triton X-100 (Sigma-Aldrich, X100), 0.1% Tween-20 (Sigma-Aldrich, P1379) in PBS). All heart and ganglion samples were incubated with primary antibody in blocking solution (1:1,000 rabbit anti-RFP, Rockland, 600-401-379; 1:1,000 chicken anti-GFP, Aves Labs, GFP-1020; 1:1,000 chicken anti-PGP9.5, Novus Biologicals, NB110-58872; 5 μg ml−1 goat anti-HB-EGF (human), R&D Systems, AF-259-NA), and secondary antibody in blocking solution (1:500 donkey anti-rabbit Cy3, Jackson ImmunoResearch, 711-165-152; 1:500 donkey anti-chicken 647, Jackson ImmunoResearch, 703-605-155; 1:500 donkey anti-chicken 488, Jackson ImmunoResearch, 703-545-155; 1:500 anti-goat 647, Jackson ImmunoResearch, 705-605-147) for 3 days at 37 °C. Stained samples were imaged by confocal microscopy (Nikon Ti2).
Whole-nerve electrophysiology
Whole-vagus-nerve electrophysiology recording was performed in anaesthetized mice (1.5–2% isoflurane in oxygen) as previously described with modifications2,18,38. In brief, the left vagus nerve was exposed and then transected below the SLN branching point. The nerve stump proximal to the heart was desheathed and placed onto a bipolar electrode (platinum–iridium). A ground electrode was placed on nearby muscle, and the nerve was immersed in halocarbon oil. The nerve activity was amplified (CP511, Grass Technologies), digitized (MP160, Biopac) and recorded (AcqKnowledge software, Biopac). Serotonin (1 mM, 50 µl, saline) was infused (intravenously) at the end of every experiment. Stimulus-induced responses were calculated as the percentage change from the baseline activity and normalized to the serotonin response. For serotonin responses (Fig. 3b) and nerve responses after blood withdrawal (Fig. 3g), nerve activity was integrated over a moving window (serotonin: 5 s, Fig. 3g: 5 ms) using the root-mean-squared method.
PV loop measurement
Left ventricular pressure and volume were measured using a Millar PV catheter (SPR-839) inserted into the carotid artery and advanced into the left ventricle. Data were acquired on the Millar MPVS-300 system with the LabChart software. After the measurements, volume calibration was performed with injection of hypertonic saline into the jugular vein and dip-well cuvettes. Analysis was performed with PV loop module in LabChart.
Optogenetics
Optogenetics experiments were performed as described with minor modifications16,18,38. The vagus nerve trunk was exposed near the carotid bifurcation, and isolated from the carotid artery and jugular vein. The nerve was transected below the SLN close to the heart above the thoracic wall, and the nerve stump proximal to the brain was illuminated to exclude motor efferent contribution (10 mW, 1 min, 10 ms pulse duration, frequencies indicated, controlled by a pulse train generator from Prizmatix). For ECG entrainment, real-time peak detection of the ECG signal (OpenEphys, crossing detector module) was coupled to optic fibre illumination at the P and QRS waves.
Two-photon calcium imaging
In vivo imaging of vagal ganglia was performed using an Olympus FVMPE resonant-scanning two-photon microscope equipped with a piezoelectric objective Z-stepper (P-915, Physik Instrumente) as previously described with minor modifications18,23. In brief, mice were anaesthetized with urethane (2 mg per g, intraperitoneal, at least 30 min before surgery) and tracheostomized to allow constant low-level air flow (40 ml min−1; 100% oxygen) delivered by a ventilator in freely breathing mice. The left vagal ganglion was surgically exposed with branches superior to the ganglion and trunk transected, and immobilized on an imaging platform attached to a manipulator. Airway stretch was achieved by increasing airflow through a ventilator (15–25 ml min−1 g−1 body weight, 5 s). For blood volume manipulation, intravenous saline was administered in the jugular vein (0.9% NaCl, 30% of total blood volume estimated by body weight or 24 µl saline infused per g body weight). Saline was administered slowly over 20 s, volume was held for 30 s and then an equal blood volume was withdrawn over 1 min. The number of healthy neurons imaged was defined by responses to stepwise electrical stimulation of the cervical vagus nerve trunk (2 ms pulses at 5 Hz over around 5 s; Grass Stimulator) of increasing current (stepwise from 0.1 mA to 5 mA). Two-channel images were processed, analysed and classified as previously described using Fiji ImageJ and Microsoft Excel18. Non-responsive neurons were selected for inclusion in heat maps based on computer randomization.
Data analysis
Haemodynamic quantification
Haemodynamic signals were analysed using custom scripts in MATLAB (MathWorks). The mean arterial blood pressure (BP) was computed from systolic (SBP) and diastolic (DBP) pressures as BP = (1/3 × SBP) + (2/3 × DBP). The heart rate (HR) was derived from beat-to-beat intervals, either from R–R intervals when ECG was available, or from the intervals between successive systolic peaks of the arterial pressure waveform when ECG was unavailable. BP and HR traces were then smoothed with a 3 s moving window, and sometimes downsampled and smoothed with a 30 s moving window for long recordings. For tilt-table experiments, each mouse underwent at least three prone-to-upright tilt trials, and each tilt onset was determined by the onboard accelerometer. The BP and HR were temporally aligned to tilt onset, and trials from multiple tilts were averaged. The baseline was the mean value during the 5 s preceding tilt. The initial BP response was defined as the maximum deviation within the first <5 s after tilt; the recovered response was the 5 s mean at 1 min after tilt. BP recovery (%) was calculated as 100 × (Δinitial BP − Δrecovered BP)/Δinitial BP, where Δ denotes the change from the baseline. For tail-bleeding experiments, BP and HR were quantified as the mean response after tail transection, expressed relative to the pretransection baseline.
Anatomical quantification
Nerve fibre innervation was quantified in confocal images (maximum intensity projection) of whole atria and ventricle slices. Nerve fibres were assigned by image segmentation using Ilastik (Ilastik, Pixel Classification) and smoothened (Otsu’s method). Nerve fibre skeletons were created using scikit-image (Python v.3.9), and nerve terminal locations were defined as branch endpoints of the skeletons. Data were curated to remove noise, including small objects (<1,500 µm2) and short branches (<17 µm). Terminal structures (defined within an 86 µm × 86 µm square centred around branch end points) were classified as end-net and flower-spray endings using a machine-learning program (Ilastik, Object Classification) after manual entrainment. A relative distribution of terminal types was calculated using a denominator of total terminals identified in a given mouse.
Nerve signal quantification
Whole-nerve electrophysiology recordings were analysed using custom-written MATLAB codes. Spikes were identified using a derivative method in which a time-shifted (250 µs) trace was subtracted from the original trace to identify acute changes in activity (events) of which the magnitude was over three times the s.d. of activity in the entire trace. Once events were identified, the difference between the minimum and maximum values in the original trace within 500 µs of the event was defined as event amplitude. An additional noise-exclusion threshold was applied to include only events of which the amplitude was over four times the s.d. of the activity in the original trace.
Removal of breathing-coupled responses in nerve recordings
The vagus nerve trunk responds both with every heartbeat and with every breath. To isolate heartbeat-coupled responses, breathing was measured using a pressure transducer within the isoflurane delivery device. The breathing trace was smoothened over a rolling 2 s window, peak inspiratory and expiratory events were identified, and inter-breath intervals (IBIs) calculated. The breathing window was defined to start 10% IBI before peak expiration and end 30% IBI after peak expiration, and all spike events within the breathing window were excluded from further analysis.
Temporal alignment to ECG recordings
To control for variations in cardiac cycle duration during nerve recordings and physiological measurements, the time axis of each cardiac cycle was normalized to the PR interval.
UMAP plots
UMAP plots were generated by analysis of published single-cell transcriptomic data of vagal sensory neurons16 using Seurat in R.
Statistics and reproducibility
Data in the graphs are presented as the mean ± s.e.m. unless otherwise indicated. Statistical analyses were performed using Prism (GraphPad), and statistical tests and sample sizes are reported in figures and legends. All replicates were biological and statistical tests were two-sided. Sample sizes were chosen based on previous studies of the vagus nerve23,38.
Materials availability
All reagents that are not commercially available will be made freely available on reasonable request.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.