General methods
DNA amplification was conducted by PCR using Phusion U Green Multiplex PCR Master Mix (ThermoFisher Scientific) or Q5 Hot Start High-Fidelity 2× Master Mix (New England BioLabs) unless otherwise noted. DNA oligonucleotides were obtained from Integrated DNA Technologies. Plasmids expressing epegRNAs were constructed by Gibson assembly using a custom acceptor plasmid. Sequences of sgRNA and epegRNA constructs used in this work are listed in Supplementary Table 1. All vectors for mammalian cell experiments were purified using Plasmid Plus Midiprep kits (Qiagen) or PureYield plasmid miniprep kits (Promega), which include endotoxin removal steps. All experiments using live animals were approved by the Broad Institute Institutional and Animal Care and Use Committees. Wild-type C57BL/6 mice were obtained from Charles River (027).
General mammalian cell culture conditions
Cell lines with homozygous PTCs in TPP1, HEXA and NPC1were generated using prime editing. HEK293T (ATCC CRL-3216), Neuro-2a (ATCC CCL-131) and HeLa (CCL-2) cells were purchased from ATCC and cultured and passaged in Dulbecco’s Modified Eagle’s Medium (DMEM) plus GlutaMAX (ThermoFisher Scientific), supplemented with 10% (v/v) fetal bovine serum (Gibco, qualified). All cell types were incubated, maintained, and cultured at 37 °C with 5% CO2. Cell lines were authenticated by their respective suppliers and tested negative for mycoplasma.
Generation of cell lines
HEK293T cells were seeded at 100,000 cells per well on 24-well plates (Corning). 16–24 h after seeding, cells were transfected at approximately 60% confluency with 600 ng, 200 ng and 60 ng of PEmax plasmid, epegRNA plasmid and ngRNA plasmid, respectively using Lipofectamine 3000 according to manufacturer’s instructions (Thermo Fisher Scientific). 4 d after transfection, single cell clones were isolated by limiting dilution cloning and expanded over a 2-week period. The resulting colonies were further expanded and genotyped by high-throughput sequencing of the targeted locus and those found to be homozygous for the expected edit were retained for downstream experiments.
Lentiviral production
HEK293Ts (ATCC CRL-3216) were transfected with pMD2.G (Addgene #12259) and delta8.2 (Addgene Plasmid #8455) packaging plasmids alongside the appropriate lentiviral backbone using Lipofectamine 2000. Lentiviral backbone sequences used in this work are listed in Supplementary Table 1. Medium was changed 24 h after transfection. Virus-containing supernatant was collected and filtered through a 0.45-μM filter 48 h after transfection. Virus was used immediately or stored at 4 °C for up to 1 week before cell transduction.
General high-throughput lentiviral screening protocol
All oligonucleotides for high-throughput screening were ordered from Twist Biosciences as single-stranded oligonucleotide pools. Oligonucleotide pools were amplified using Q5 Hot Start High-Fidelity 2X Master Mix (NEB M0494L) to create double-stranded inserts for isothermal assembly. Primers used are indicated in each specific screening section of the Methods. The minimum number of PCR cycles was used to amplify (typically between 11 and 13 cycles) and double-stranded inserts were checked for size and/or inappropriate products by TapeStation (Agilent). For each 20-μl reaction cloned into the pSEP0308, pSEP0309 or pSEP0310 lentiviral backbones with a 300-bp insert, 50 ng of the appropriate lentiviral backbone was assembled with 10 ng of the appropriate double-stranded insert using NEBuilder HiFi DNA Assembly Master Mix (NEB E2621L). Reactions were scaled according to the number of elements in the library. For each 1,000 elements, an additional 20-μl reaction was set up. Reactions were incubated at 50 °C for 2 h and then pooled and purified using the QIAquick PCR purification kit (Qiagen 28104) according to manufacturer’s instructions. Reaction products were eluted in a minimum of 20 μl ddH2O, but otherwise were eluted in 2.5 μl per original 20-μl reaction.
Isothermal assembly reactions were electroporated into NEB 10-beta Electrocompetent Escherichia coli (NEB C3020) and plated onto LB plates aiming for at least 1,000× coverage of each library element. 14 h later, colonies were scraped and prepared using Qiagen Plasmid Plus kits (Qiagen 12945 and 12963) according to manufacturer’s instructions. Pooled plasmids were transfected into HEK293Ts alongside lentiviral packaging plasmids using Lipofectamine 2000. Medium was changed 24 h after transfection. Virus-containing supernatant was collected and filtered through a 0.45-μM filter 48 h after transfection. Cells were transduced with virus aiming for a multiplicity of infection of 0.3. Cells were transduced to ensure ≥1,000× coverage of transduced cells per element of the library. 2 d after transduction, cells were passaged with 1 μg ml−1 puromycin for 3–4 days to enrich for transduced cells. For screens requiring FACS isolation of GFP-positive cells, we calculated approximate coverage by multiplying the per cent of GFP-positive cells by the number of elements in the library and aiming for 1,000× coverage of that number. For example, if 10% of cells were GFP-positive for a 500 element library, we sorted at least 50,000 cells per replicate. Cells were pelleted and flash frozen on dry ice. Genomic DNA was isolated using QIAamp DNA Micro kits (Qiagen 56304) or QIAamp Mini kits (Qiagen 51304) depending on the cell number isolated.
All genomic DNA for each replicate was input into an initial set of PCR reactions using Q5 Hot Start High-Fidelity 2X Master Mix (NEB M0494L) to amplify the integrated lentiviral cassette and to add sequencing adapters, with a maximum of 5 μg genomic DNA per 50-μl reaction. Primers used are indicated in each screening section and are also listed in Supplementary Table 1. PCR reactions were purified using the QIAquick PCR purification kit (Qiagen 28104) according to manufacturer’s instructions and 2 μl of PCR product was input into a second PCR reaction to add unique sample indices and flow cell adapters to each amplicon. Final PCR reactions were bead purified with a ratio of 0.7× using SPRI beads, quality controlled using a TapeStation (Agilent) and quantified using a Qubit prior to high-throughput sequencing on an Illumina MiSeq, Illumina NextSeq, or Element Biosciences AVITI instrument. Sequencing conditions and downstream analyses are specific to each screen and are indicated in each separate screening section.
PE2 screening
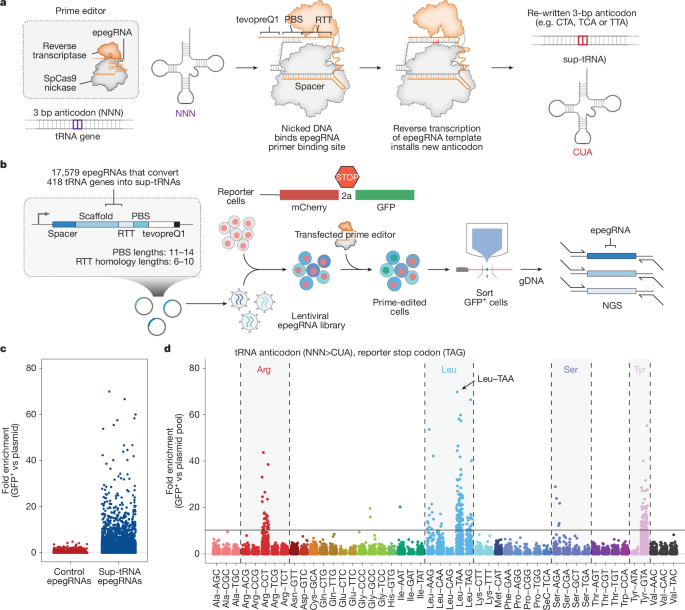
To convert each endogenous tRNA into a sup-tRNA, we identified 418 high-confidence tRNA genes and identified the two closest 20-bp spacers with an NGG protospacer-adjacent motif upstream and downstream of the anticodon. We allowed prime editing to target multiple tRNAs in the case of identical mature tRNA sequences. We systematically varied the PBS lengths from 11 bp to 14 bp, and varied the RTT homology lengths 3′ of the last edited nucleotide from 6 bp to 10 bp, where feasible. We designed three prime editing screens, each with RTTs that replaced the anticodon of each endogenous tRNA with one of three sup-tRNA anticodons (CUA, UCA or UUA). To enhance prime editing efficiency, we appended a structured RNA motif, tevopreQ1, to the 3′ end of each pegRNA, creating epegRNAs29. In total, we designed 17,579 epegRNAs to convert endogenous tRNAs to sup-tRNAs for each of the three possible sup-tRNA anticodons. Each pool also included 420 control epegRNAs targeting serine and arginine tRNAs, swapping their anticodons for those of other serine and arginine anticodons, which are not expected to give signal in a readthrough-based screen (Supplementary Table 2). We included a unique barcode sequence 3′ of the polyT terminator sequence for each epegRNA to improve our ability to assign each sequencing read to the correct element. The final oligonucleotides had the following design: a common 5′ end for isothermal assembly (5′-tatcttgtggaaaggacgaaacacc-3′), a unique epegRNA sequence followed by a polyT for Pol III termination, an adjustable length linker used to ensure that each element of the library was the same length, a unique 25 bp barcode, and a common 3′ end for isothermal assembly (5′-ctcgagtactaggatccattaggcg-3′). Oligonucleotides were amplified using oSEP0114 and oSEP0115 prior to isothermal assembly into a linearized pSEP0308 with a hU6 promoter for driving expression of the epegRNAs. Final libraries for next generation sequencing were sequenced on an Illumina MiSeq instrument using oSEP0213 as a custom Read1 primer with 300 cycles. For analysis, the first 25 cycles of the read were matched exactly to the barcode for each element of the screening pool, allowing for no mismatches. Reads were normalized on the basis of sequencing depth and sorted samples were compared with the plasmid pool representation of each element. Oligonucleotides and results for this screen are listed in Supplementary Table 2.
Lentiviral sup-tRNA screen
To evaluate a wide range of tRNA promoter variants in high-throughput, we took the 418 high-confidence tRNA sequences from the human genome and swapped their anticodon to CUA, UCA or UUA to target the stop codons TAG, TGA or TAA, respectively. A unique barcode was assigned to each tRNA variant to reduce the likelihood that sequencing errors would influence assignment of library members. In addition, a pre-integrated Nextera Read1 adapter was included so that Read1 sequences on an Illumina instrument will read straight into the highly complex barcode for defining clusters. We cloned this pool using isothermal assembly into three different lentiviral backbones to evaluate the effect of different upstream elements on sup-tRNA efficacy: pSEP0308, which has a hU6 promoter, pSEP0309, which has a minU6 promoter and pSEP0310, which contains no exogenous promoter. For analysis, the first 25 cycles of the read were matched exactly to the barcode for each element of the screening pool, allowing for no mismatches. Reads were normalized on the basis of sequencing depth and sorted samples were compared with the plasmid pool representation of each element. Oligonucleotides and results for this screen are listed in Supplementary Table 3.
Leader and terminator sequence screening
For the initial leader sequence screen, the 40 bp upstream of each endogenous high-confidence tRNA in the human genome was determined and placed upstream of the mature sequence for tRNA-Leu-TAA-3-1 with its anticodon changed to CUA followed by a polyT termination sequence. As controls, the same leader sequences were placed upstream of the mature sequence for tRNA-Leu-TAA-3-1 with its native anticodon. Libraries were sequenced and exact matching was used for both the leader sequence and the anticodon region of the read to assign to the correct library element. Oligonucleotides and results for this screen are recorded in Supplementary Table 4.
To next screen leader sequences across a wide range of sup-tRNAs, we generated a lentiviral library containing six leader sequences (two top-performing, two bottom-performing and two random sequences) paired with 418 human tRNAs with their anticodons switched to CUA, UCA or UAA. We also assessed the effect of each terminator sequence when placed downstream of sup-tRNAs paired with their endogenous leader sequences, with a total of 11,543 combinations of leader sequence, sup-tRNA and terminator. We included a unique 20-bp barcode sequence 3′ of the terminator sequence for each tRNA to improve our ability to assign each sequencing read to the correct element. For analysis, the first 25 cycles of the read were matched exactly to the barcode for each element of the screening pool, allowing for no mismatches. For analysis, alignment was confirmed between tRNA elements and the 20-bp barcode region and then subsequently the 20-bp barcode was used to assign each read to the appropriate library element. Reads were normalized on the basis of sequencing depth and sorted samples were compared with the plasmid pool representation of each element. Oligonucleotides and results for this screen are recorded in Supplementary Table 5.
Saturation mutagenesis screening
To design variants for saturation mutagenesis screening, every sequence containing a SNV, paired substitution at all hairpin positions, and 1-bp deletion was generated computationally. Then, 25 bp ends compatible with isothermal assembly were added to either side (see Supplementary Table 1) and a unique barcode was assigned to each tRNA variant to reduce the likelihood that sequencing errors would influence assignment of library members. In addition, a pre-integrated Nextera Read1 adapter was included so that Read1 sequences on an Illumina instrument will read straight into the highly complex barcode for defining clusters. Variants were ordered as a Twist oligonucleotide pool for each individual tRNA for tRNA-Leu-TAA-4-1, tRNA-Arg-CCT-4-1, mouse tRNA-Leu-TAA-2-1 and tRNA-Tyr-GTA-2-1 (sequences in Supplementary Table 6). A pooled library of all possible variants for tRNA-Leu-TAA-1-1, tRNA-Leu-TAA-2-1, tRNA-Leu-TAA-3-1, and tRNA-Leu-TAA-4-1 was ordered as a separate Leu-TAA focused library (sequences in Supplementary Table 7).
For the tRNA-Arg-CCT-4-1, mouse tRNA-Leu-TAA-2-1, and tRNA-Tyr-GTA-2-1 screens, Twist oligonucleotides contained a leader sequence element for each tRNA and they were cloned by isothermal assembly into the pSEP0310 lentiviral backbone with no exogenous Pol III promoter. For the tRNA-Leu-TAA-4-1 and Leu-TAA focused library, Twist oligonucleotides did not contain a leader sequence element for each tRNA and they were cloned by isothermal assembly into the pSEP0308 lentiviral backbone with an exogenous hU6 promoter driving expression of the tRNAs. Libraries were sequenced on an Illumina instrument with 26 cycles for Read1 covering the barcode region and 86 cycles for Read2 covering the tRNA region. For analysis, alignment was confirmed between tRNA elements and the 25-bp barcode region and then subsequently the 25-bp barcode was used to assign each read to the appropriate library element.
epegRNA optimization with synthetic target-site screening
To optimize epegRNA architectures, we designed a lentiviral epegRNA library of 17,280 epegRNAs to test out five spacer variants, PBS lengths from 8 to 16 nt, RTT lengths from 21 to 36 nt, and combinations of each of the 19 mutation variants of interest alongside 720 control epegRNAs with RTTs that do not encode an anticodon edit. On the same oligonucleotide, we encoded an adjacent tRNA-Leu-TAA-1-1 synthetic target site directly adjacent to the lentiviral epegRNA to read out both the epegRNA and the outcome of editing in the same sequencing read. We transfected cells transduced with this library with a panel of prime editor proteins that included PEmax and several engineered PE6 variants40 listed in Supplementary Data Tables 9 and 10. We performed experiments in both MMR-deficient (HEK293T) cells and MMR-proficient (HeLa) cells and included conditions in each case in which MMR was transiently inhibited by co-transfection of a dominant-negative MMR protein (MLH1dn)38. For experiments in HeLa cells, we collected genomic DNA 3 d after transfection. For experiments in HEK293T cells, we collected genomic DNA at 3 d and 9 d after transfection. We then performed high-throughput sequencing of the lentivirally integrated cassette. For analysis, sequencing reads were initially demultiplexed into individual fastq files by first aligning each read to a corresponding member of the epegRNA library using bowtie2 (ref. 65). Next, each individual fastq file was then trimmed to only include the full-length synthetic target site. Editing outcomes for each epegRNA sequence were quantified using CRISPResso2. Oligonucleotides and results for this screen are recorded in Supplementary Data Tables 9 and 10.
RNA-seq and transcriptomic analysis
HEK293T cells were transfected with our optimized epegRNA sequence and ngRNA alongside PE6c or with an unrelated epegRNA and ngRNA pair targeting the HEK3 locus alongside PE6c (see Supplementary Table 1). RNA was extracted 6 d after transfection using the Qiagen RNeasy kit (Qiagen) according to manufacturer’s instructions. RNA-seq libraries were generated using the SMART-Seq mRNA LP kit (Takara Bio) according to manufacturer’s instructions and sequenced 2× 75-bp on an Element Biosciences AVITI instrument. Fastq reads were trimmed of adapter sequences using Trim Galore, aligned to the human genome using STAR, and differential expression analysis was performed using DESeq2 and custom R scripts.
Measurement of tRNA abundance
Total RNA, including small RNAs, was isolated from prime edited cells using miRNeasy kits (Qiagen) according to manufacturer’s protocols. RNA was reversed transcribed with SuperScript IV (Thermo Fisher Scientific) using a primer specific to each tRNA gene family queried66 (Supplementary Table 1). Next, quantitative PCR was performed using Power SYBR Green PCR master mix (Thermo Fisher Scientific) and primers specific to each tRNA gene family queried (Supplementary Table 1). For tRNA-Leu-TAA-1-1, we performed targeted tRNA sequencing. Specifically, we measured the relative abundance of the desired tRNA edit versus unedited in a polyclonal edited population in both genomic DNA and total RNA. For targeted tRNA sequencing, we performed reverse transcription using a primer specific to tRNA-Leu-TAA-1-1 and the Induro RT enzyme that is tolerant to RNA modifications39.
Sequence context screening
To generate a diverse PTC sequence context reporter library, 14,746 naturally occurring premature TAG stop codons in protein-coding genes were identified in ClinVar that were annotated as pathogenic, likely pathogenic, or of uncertain significance. Each TAG stop codon was flanked on either side by the 18 nucleotides present in the native mRNA sequence. Positive controls were generated that contained the same sequence context but a TTG Leucine codon instead of a TAG premature stop codon. For negative controls, 2,800 ‘redundant stop’ control library members were generated, which were a subset of the ClinVar variants and their TTG controls but with the codon following the TAG stop codon or TTG codon changed to a TAA stop codon followed by a +1 frameshift to prevent readthrough.
The pSEP0211 lentiviral backbone was first linearized using oSEP0163 and oSEP0164 and the oligonucleotide pool was amplified using oSEP0165 and oSEP0166 (Supplementary Table 1). These sequences were cloned into the pSEP0211 lentiviral backbone between mCherry and GFP via Gibson assembly. Two days following transduction, we extracted both mRNA and genomic DNA from the transduced cell population, generated cDNA by reverse transcription, and sequenced the integrated reporter construct from both the cDNA and genomic DNA samples. For analysis, we calculated an ‘RNA score’ metric by quantifying the frequency of each element in the cDNA and dividing that value by the frequency of that element in the genomic DNA. To control for the impact each unique sequence context might have on transcript expression level independent of readthrough activity, we defined a ‘readthrough score’ for each ClinVar PTC by dividing the RNA score of each variant by the RNA score of its corresponding no-premature-stop equivalent. Oligonucleotides and results for this screen are recorded in Supplementary Table 15.
Off-target prime editing screening
To identify candidate off-target prime editing sites, we used Cas-OFFinder67 to identify all human genomic sequences with up to six mismatches, or up to four mismatches combined with up to two bulges relative to our optimized epegRNA spacer sequence. To account for target-site binding and potential annealing of the full-length RTT product, we extracted the local sequence surrounding these sites to include 3 bp upstream of the putative target site and 34 bp downstream. We included 100 uniquely barcoded positive-control sequences corresponding to the endogenous tRNA-Leu-TAA-1-1 site and 694 negative-control sequences that shared no homology with the epegRNA spacer sequence. These sequences were cloned into the pSEP0310 lentiviral backbone and transduced into HEK293T cells after lentiviral production. These cells were then transfected with an epegRNA expression plasmid alone as a negative control, or with a PE6c prime editor expression plasmid alongside the epegRNA expression plasmid. 3 d after transfection, genomic DNA was isolated and the lentiviral integrated target site was amplified for high-throughput sequencing. The amplified target-site was sequenced using 2× 115 paired-end high-throughput sequencing. For analysis, high-throughput sequencing reads were initially demultiplexed into individual fastq files by first aligning each read to a corresponding member of the target-site library using bowtie2 (ref. 65). Next, each individual fastq file was then trimmed to only include the full-length putative off-target site. Putative off-target editing events were characterized as previously described34. In brief, the sequence encoded by the RTT was compared base-by-base to the nucleotide sequence 3′ of the cas9 nick site of each potential off-target site with an ‘off-target marker’ sequence being identified as the minimal deviating sequence between the two. The presence of this off-target marker sequence was quantified using output from CRISPResso2 and was used as a proxy for off-target prime editing. Oligonucleotides and results for this screen are recorded in Supplementary Table 11.
rhAmpSeq off-target-site amplification and analysis
For initial off-target analysis, we used Cas-OFFinder to identify all human genomic sequences with up to 4 mismatches relative to our optimized epegRNA spacer sequence. A pooled sequencing primer was generated for nominated human off-target sites using the rhAmpSeq design tool (IDT). Genomic DNA was extracted from editor-treated HEK293T cells and amplified with rhAmpSeq pooled sequencing primers according to the manufacturer’s protocol. The amplified libraries were sequenced with 300-bp single-end reads with an Illumina MiSeq. Sequences for rhAmpSeq amplicons were extracted using the R Bioconductor BSGenome package (v.1.4.3) using the GRCh37/hg19 (human) reference genomes. CRISPResso2 was used to align the rhAmpSeq reads to the amplicon reference sequences and quantify the number of reads with each possible edit.
Flow cytometry
Cells were trypsinized, resuspended in media containing 10% FBS, and the solution was filtered through a 45-μm cell strainer prior to flow cytometry or FACS isolation. Flow cytometry analysis was performed using the CytoFLEX LX Flow Cytometer (Beckman Coulter, C06779) at the Broad Institute Flow Cytometry Core, with CytExpert Acquisition and Analysis Software (v.2.4). FACS was performed on the SONY MA900 Cell Sorter (Sony Biotechnology) and cells were sorted into medium prior to being spun down.
General cloning
Plasmid vectors for mammalian expression of epegRNAs or ngRNAs were cloned via isothermal assembly as previously described67. In brief, a human U6 promoter vector was linearized via polymerase chain reaction and incubated with IDT eBlocks encoding the full-length epegRNA or ngRNA sequence flanked by sequences necessary for isothermal assembly using NEBuilder HiFi DNA Assembly Master Mix (New England BioLabs). A list of epegRNA and ngRNA sequences used in this study are provided in Supplementary Table 1.
AAV vector genomes were cloned via isothermal assembly as previously described50. In brief, the v3em vector genome construct (Addgene, #198735) was linearized by restriction digest and new epegRNA and ngRNA sequences, encoded across two IDT eBlocks, were inserted via isothermal assembly using NEBuilder HiFi DNA Assembly Master Mix (New England BioLabs). The PE6d and PE6e v3em vectors were assembled via isothermal assembly using NEBuilder HiFi DNA Assembly Master Mix (New England BioLabs). The sequences of interest were PCR amplified out of existing vectors (Addgene, #207854 and 207855) and assembled into the corresponding v3em vector (Addgene, #198735 and #193734).
Arrayed genome-editing experiments
For all experiments performed in HEK293T or HeLa readthrough reporter polyclonal cell lines and experiments performed in Neuro-2a cells, cells were seeded at 12,000 cells per well into 96-well plates (Corning) and transfected the following day with Lipofectamine 3000 (Thermo Fisher Scientific). A total of 200 ng, 66 ng and 22 ng of prime editor plasmid, epegRNA plasmid and ngRNA plasmid (where indicated), respectively, were transfected per well. 3 d after transfection, cells were lysed and genomic DNA was collected by incubation in a lysis buffer containing 10 mM Tris-HCl, pH 8.0, 0.05% SDS and 800 units per μl of proteinase K (New England BioLabs) at 37 °C for 1 h, followed by enzyme inactivation at 80 °C for 30 min.
For rescue experiments performed in the HEK293T disease models, cells were initially seeded at 200,000 cells per well in 12-well plates (Corning). The following day, cells were transfected with 700 ng of PE6c prime editor plasmid, 200 ng of epegRNA plasmid and 75 ng of ngRNA plasmid using Lipofectamine 3000 (Thermo Fisher Scientific). 3 d after transfection, genomic DNA was extracted as described above.
Protein isolation and enzymatic activity assays
For assays using human cells, cells were collected by trypsinization, pelleted, washed in 1× phosphate-buffered saline and repelleted. For the tripeptidyl peptidase (TPP1) assay, pelleted cells were lysed at 4 °C for 30 min in a buffer containing 0.1% Triton X-100 (Sigma) and 10% SDS (Thermo Fisher Scientific) in 1× phosphate-buffered saline. For the hexosaminidase (HEXA) assay, pelleted cells were lysed in a RIPA homogenizing buffer supplemented with a protease inhibitor cocktail (Roche) at 4 °C for 30 min. In both cases, lysates were then centrifuged at 20,000g for 20 min at 4 °C. Supernatant was collected and total protein concentration was quantified using the Pierce BCA protein assay kit according to the manufacturer’s protocol (Thermo Fisher Scientific). For both HEXA and TPP1 assays, the sensitivity and accuracy of the assay was determined by a standard curve derived from measurements of known quantities of wild-type protein lysate (Supplementary Fig. 10b,c).
For the TPP1 assay, 10 μg of protein lysate was incubated at 37 °C overnight in a 0.1 M sodium acetate buffer at pH 4 containing the Ala-Ala-Phe-7-amido-4-methylcoumarin substrate (Sigma, A3401) at a final concentration of 250 μM in a reaction volume of 40 μl. Endpoint fluorescence was then measured with a Tecan Spark Multimode Microplate Reader with an excitation wavelength of 360 nm (20 nm bandwidth) and emission of wavelength of 460 nm (20 nm bandwidth). Enzymatic activity relative to wild-type was calculated by dividing the mean fluorescence values from each treatment condition by the mean fluorescence values of mock-treated wild-type HEK293T cells.
For the HEXA assay, 5 μg of protein lysate was incubated for 1 h at 37 °C in a 0.1 M citrate phosphate buffer at pH 4.5 containing either 4-methylumbelliferone-f-N-acetylglucosamine (MUG, Sigma, 69585) or 4-methylumbelliferone-O–N-acetylglucosamine-6-sulfate (MUGS, Sigma, 454428) substrate at a concentration of 3.2 mM. Each reaction was carried out in a total volume of 50 μl and stopped by the addition of 200 μl of a 100 mM solution of 2-amino-2-methyl-1-propanol. Endpoint fluorescence measurements were made with a Tecan Spark Multimode Microplate Reader with an excitation wavelength of 360 nm (20 nm bandwidth) and emission of wavelength of 450 nm (20 nm bandwidth). HEXA activity was normalized to HEXB activity by dividing the mean fluorescence values from the MUGS (HEXA) reaction by the corresponding mean fluorescence values from the MUG (HEXB) reaction. Enzymatic activity relative to wild-type was calculated by dividing the derived mean HEXA activity values for each treatment condition by that of the mean HEXA activity values from mock-treated wild-type HEK293T cells.
For measurements of α-L-iduronidase in mouse tissue, protein was extracted by first homogenizing the tissue using the TissueLyser II (QIAGEN) in T-PER tissue protein extraction reagent (Thermo Fisher Scientific, 78510) containing cOmplete Protease Inhibitor Cocktail (Roche). Lysates were then centrifuged at 20,000g for 20 min at 4 °C and supernatant was collected and total protein concentration was quantified using the Pierce BCA protein assay kit according to the manufacturer’s protocol (Thermo Fisher Scientific). Up to 40 μg of whole protein lysate was incubated overnight in a 130 mM sodium formate buffer containing 0.42 mg ml−1 of d-saccharic acid 1,4-lactone monohydrate (Sigma-Aldrich, S0375) and 4MU-iduronic acid (0.12 mM; Santa Cruz Biotechnology, sc-220961) at pH 3.5. Endpoint fluorescence measurements were made with a Tecan Spark Multimode Microplate Reader with an excitation wavelength of 365 nm (20 nm bandwidth) and emission of wavelength of 450 nm (20 nm bandwidth).
Western blotting
Cells were collected by trypsinization, pelleted, washed in 1× phosphate-buffered saline then lysed in a RIPA homogenizing buffer supplemented with a protease inhibitor cocktail (Roche) at 4 °C for 30 min. Lysates were then centrifuged at 20,000g for 20 min at 4 °C. Supernatant was collected and total protein concentration was quantified using the Pierce BCA protein assay kit according to the manufacturer’s protocol (Thermo Fisher Scientific). Up to 10 μg of whole protein lysate was separated by SDS–PAGE using a 4–12% Bolt Bis-Tris Plus Mini Protein Gel (Invitrogen). Proteins were transferred to a nitrocellulose membrane using the iBlot 2 Dry Blotting system (Thermo Fisher Scientific) then incubated in a 5% milk solution in 1 × Tris-Buffered Saline, 0.1% Tween-20 (TBST). The blocked membrane was incubated at 4 °C overnight in NPC1 (Abcam, ab134113, 1:2,500 dilution) or GAPDH (Santa Cruz, sc-47724, 1:5,000 dilution) primary antibody. The following day, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Abcam, ab6721; 1:10,000 dilution) for NPC1 and anti-mouse IgG (Abcam, ab205719; 1:10,000 dilution) for GAPDH for 1 h at room temperature. The secondary antibody was removed and the membrane was washed and signal was collected using the SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Protein isolation, trypsin digestion and TMT labelling for mass spectrometry
Protein was isolated as described for Western blotting. To prepare samples for mass spectrometry, samples were passed over S-trap micro spin columns (Profiti) according to manufacturer’s protocols with the following modifications: 10 mM DTT (final concentration) was used instead of TCEP. After adding DTT, tubes were placed on a heating block for 10 min at 95 °C. Disulfides were alkylated with 20 mM iodoacetamide (final concentration) instead of methyl methanethiosulfonate, and after adding the iodoacetamide the samples were incubated at 25 °C for 30 min in the dark. After digestion with 5 µg trypsin (Thermo Fisher 90057), samples were desalted using Pierce Peptide Desalting Spin Columns (Thermo Fisher 89852) following the manufacturer’s protocol. The desalted tryptic peptides were resuspended in 100 µl of 100 mM triethylammonium bicarbonate buffer (TEAB), vortexed, and briefly centrifuged. For labelling with tandem mass tags (TMTs), lyophilized TMTpro Label reagents (Thermo Fisher A44520) were used according to manufacturer’s protocols. At the time of labelling, aliquots were equilibrated at room temperature and 20 µl of anhydrous acetonitrile was added to each tube. The TMT reagents were vortexed, briefly centrifuged, and allowed to dissolve for 5 min at 25 °C. TMT reagents were added to each 100-µl sample, vortexed, and briefly centrifuged. The samples were incubated for 1 h at 25 °C. After 1 h, 5 µl of 5% hydroxylamine was added to each sample and incubated for 15 min to quench the reaction. Equal amounts of each labelled sample were then combined together and speed-vacuumed to dryness.
LC–MS
The TMT-labelled tryptic peptides were separated by reverse phase HPLC (Thermo Ultimate 3000) using a Thermo PepMap RSLC C18 column (2 µm tip, 75 µm x 50 cm ES903) over a gradient before nano-electrospray using a Orbitrap Exploris 480 mass spectrometer (Thermo). Solvent A was 0.1% formic acid in water and solvent B was 0.1% formic acid in acetonitrile. The mass spectrometer was operated in a data-dependent mode. The parameters for the full scan MS were: resolution of 60,000 across 450–1,600 m/z and maximum IT 50 ms. The full mass spectrometry scan was followed by MS/MS for as many precursor ions in a three-second cycle with a NCE of 32, dynamic exclusion of 30 s and resolution of 45,000.
Database search with Proteome Discoverer
Raw mass spectral data files (.raw) were searched using Sequest HT in Proteome Discoverer (Thermo). Sequest search parameters were: 10 ppm mass tolerance for precursor ions; 0.02 Da for fragment ion mass tolerance; 2 missed cleavages of trypsin. Fixed modifications were carbamidomethylation of cysteine and TMTpro modification on lysines and peptide N termini. Variable modifications were methionine oxidation, methionine loss at the N terminus of the protein, acetylation of the N terminus of the protein, and methionine loss plus acetylation of the protein N terminus.
AAV production
AAV production was performed using HEK293T clone 17 cells (ATCC, CRL-11268) maintained in DMEM plus GlutaMAX (Thermo Fisher Scientific) with 10% heat-inactivated FBS without antibiotic in 150 mm2 dishes (Thermo Fisher Scientific). The day before transfection cells were plated at a density of 18 million cells per 150 mm2 plate. The following day, 5.7 μg of AAV genome plasmid, 11.4 μg of pHelper (Clontech) and 22.8 μg of rep-cap plasmid per plate were delivered via polyethyleneimine transfection (PEI MAX, Polysciences). Three days after transfection, cells were collected by cell scraping and pelleted at 3,000g for 10 min. Medium was decanted into a solution of poly(ethylene glycol) (PEG) 8000 (Sigma-Aldrich) and NaCl at a final concentration of 8% PEG and 500 mM NaC and incubated on ice for 2 h. The cell pellet was resuspended in 500 µl per plate of a hypertonic buffer containing 40 mM Tris base, 500 mM NaCl, 2 mM MgCl2 and 100 U ml−1 salt active nuclease (ArcticZymes) and incubated for one hour at 37 °C. The medium-containing solution was then pelleted by centrifugation at 3,000g for 30 min and the resulting pellet was resuspended in 500 µl per plate of a hypertonic lysis buffer and combined with the cell lysate. This lysate was then added to Beckman Coulter Quick-Seal tubes via 16-gauge, 5-inch needles (Air-Tite N165) in a discontinuous gradient of iodixanol in sequentially floating layers as follows: 9 ml of 15% iodixanol in 500 mM NaCl and 1× phosphate-buffered saline-MK (1× phosphate-buffered saline plus 1 mM MgCl2 and 2.5 mM KCl), 6 ml of 25% iodixanol in 1× phosphate-buffered saline-MK and 5 ml each of 40% and 60% iodixanol in 1× phosphate-buffered saline-MK with phenol red at a concentration of 1 μg ml−1 in the 15%, 25% and 60% layers to aid layer visualization This gradient was then ultracentrifuged using a fixed-angle Ti 70 rotor in an Optima XPN-100 Ultracentrifuge (Beckman Coulter) at 68,000 rpm for one hour at 18 °C. Next, 3 ml of virus-containing solution was extracted from the 40–60% iodixanol interface via an 18-gauge needle. Buffer was exchanged for cold phosphate-buffered saline with 0.001% F-68 using a PES 100 kD MWCO column (Thermo Fisher Scientific) and concentrated. The resulting AAV-containing solution was sterile filtered using a 0.22-μm filter and quantified by quantitative PCR (qPCR) (AAVpro Titration Kit version 2, Clontech). Purified virus was stored at 4 °C until use.
Animal use
All experiments involving live animals were approved by the Broad Institute Institutional Animal Care and Use Committee (D16-00903; 0048-04-15-2). Mouse housing facilities were maintained at 20–22 °C with 30–50% humidity, on a 12 h light/12 h dark cycle with ad libitum access to standard rodent diet and water. For experiments involving IduaW392X mice, we used Strain from Jackson Laboratory.
Neonatal ventricular injections
Syringes for microinjection were prepared by pulling PCR Micropipettes (Drummond Scientific Company, 5-000-1001-X10) with the Sutter P1000 micropipette puller for a tip diameter size of 100 μm. The injection solution was prepared by mixing 5 × 1010 vg of each half of prime editor encoding AAV and 1 × 1010 vg of reporter AAV in 0.9% NaCl solution (Covetrus, 061758) in total volume of 4 μl, along with 0.1 μl of Fast Green. A total of 4 μl of injection solution was front-loaded to the micropipette syringe for injection. Neonatal mice were anaesthetized on ice. A total of 2 μl of injection solution was injected into each ventricle with successful injection being verified by the spread of Fast Green dye by transillumination of the head. Litters were randomized for injection with a given AAV composition. Both sexes were included for each experimental condition for in vivo experiments. Both sexes were assigned to each experimental group as evenly as possible. No statistics were performed to pre-determine sample size for each group.
In vivo prime editing
In experiments demonstrating readthrough of an exogenous reporter co-administered via AAV, we evaluated both TAG and TGA stop codon readthrough. To achieve TGA stop codon readthrough, we used the epegRNA architecture optimized for the introduction of the CUA anticodon but modified the 3′ extension to introduce a UCA anticodon. The reporter construct contained an eGFP expression cassette with either a TAG or TGA PTC at codon 81 or the wild-type glutamine codon. In experiments evaluating the long-term tolerability of PERT, we delivered editing reagents in the v3em dual-AAV architecture introducing either an CUA anticodon into the mouse tRNA-Leu-TAA-2-1 gene, or a control +5 G-to-T edit in the Dnmt1 locus. At three weeks, the cortices and livers from mice were processed for whole proteome mass spectrometry to evaluate readthrough past natural stop codons.
Mouse tissue collection, histology and immunohistochemistry
Mice used in this study were sacrificed by CO2 asphyxiation, and unperfused tissues were immediately dissected. For protein and DNA analysis, dissected tissues were immediately frozen in liquid nitrogen. Protein extraction was performed as described above. Genomic DNA and RNA was extracted using the QIAGEN AllPrep DNA/RNA kit according to the manufacturer’s protocol.
For histology and immunohistochemistry, dissected tissues were placed in 10% neutral buffered formalin and then soaked in 70% ethanol prior to paraffin embedding. Following routine processing and paraffin embedding, tissue sections were stained with either haematoxylin and eosin, Alcian blue, or prepared for immunohistochemistry. For immunohistochemistry, 4-μm-thick sections were deparaffinized and rehydrated, followed by antigen retrieval using a sodium citrate buffer. After quenching endogenous peroxidase and application of a protein block (Dako), sections were incubated with either an anti-GFP antibody (Abcam, ab183734) or an anti-iduronidase (R&D systems, AF4119) antibody. Staining was detected using a species-specific detection kit, diaminobenzidine was used as the chromogen, and Mayer’s Haematoxylin (Dako) was used as the counterstain. Primary antibodies were substituted with an appropriate negative-control IgG for negative-control slides. For Alcian blue staining, 4-μm formalin-fixed, paraffin-embedded tissue sections were deparaffinized and rehydrated. Sections were stained with the Alcian Blue–1%, pH 2.5 kit (Newcomer, 9102A). Tissue histopathology was performed at the University of Minnesota Comparative Pathology Shared Resource and pathology scoring was performed by a board-certified veterinary pathologist blinded to the treatment conditions.
Statistics and reproducibility
All screens were performed in independent biological duplicates. Sample sizes for all other experiments and analyses are defined in the corresponding figure legends. Where performed, select comparisons are indicated within the figure, and P values can be found in corresponding Supplementary Tables.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.