In a town on the shores of Lake Geneva sit clumps of living human brain cells for hire. These blobs, about the size of a grain of sand, can receive electrical signals and respond to them — much as computers do. Research teams from around the world can send the blobs tasks, in the hope that they will process the information and send a signal back.
Welcome to the world of wetware, or biocomputers. In a handful of academic laboratories and companies, researchers are growing human neurons and trying to turn them into functional systems equivalent to biological transistors. These networks of neurons, they argue, could one day offer the power of a supercomputer without the outsized power consumption.
Can lab-grown brains become conscious?
The results so far are limited. But keen scientists are already buying or borrowing online access to these brain-cell processors — or even investing tens of thousands of dollars to secure their own models.
Some want to use these biocomputers as straightforward replacements for ordinary computers, whereas others want to use them to study how brains work. “Trying to understand biological intelligence is a very interesting scientific problem,” says Benjamin Ward-Cherrier, a robotics researcher at the University of Bristol, UK, who rents time on the Swiss brain blobs. “And looking at it from the bottom up — with simple small versions of our brain and building those up — I think is a better way of doing it than top down.”
Biocomputing advocates claim that these systems could one day rival the capability of artificial intelligence and the potential of quantum computers.
Other researchers who work with human neurons are more sceptical of what’s possible. And they warn that hype — and the science-fictional allure of what are sometimes labelled brain-in-a-jar systems — could even be counterproductive. If the idea that these systems possess sentience and consciousness takes hold, there could be repercussions for the research community.
“I’m nervous that, if this kind of work gets a lot of attention and is overstated, that the reaction won’t just be, ‘We need to think about this work a little more carefully’. It will be, ‘We need to stop this work entirely,’” says Madeline Lancaster, a developmental biologist who uses neural tissue to study development and disease at the University of Cambridge, UK, but is not involved in biocomputing projects. “That could bring in regulations that prevent all work, including on the side of the field that’s really doing research to try to help people.”
Power down
Computer scientists have long coveted the astonishing power efficiency of the human brain. Running on less than 20 watts — about enough to work a small desktop fan — its billions of neurons can spin through the equivalent of one billion billion mathematical operations each second. The best supercomputers can match that speed, but consume a million times more power in doing so.
Some researchers are trying to replicate the super-efficient structure of the brain using silicon chips. This approach, broadly called neuromorphic computing, takes inspiration from how neurons connect and fire to communicate. Specifically, some systems seek to mimic how neurons must charge to a threshold before firing an electrical impulse.
Biocomputing, on the other hand, goes back to the biological source material. Starting with induced pluripotent stem (iPS) cells, which can be reprogrammed to become almost any type of cell, researchers culture communities of brain cells and nurture them with nutrients and growth factors. To communicate with them, researchers sit the cells on electrode arrays, then pass signals and commands to them as sequences of electrical pulses. These signals change the way that ions flow into and out of neurons, and might prompt some cells to fire an electrical impulse known as an action potential. The biocomputer electrodes can detect these signals and employ algorithms to convert them to usable information.
Neurons in a dish learn to play Pong — what’s next?
The most common biocomputing approach cultures the neurons as 3D clusters called organoids. The composition of these brain-cell communities varies, depending on how the iPS cells differentiate, but typically includes neurons and cells that support them, such as astrocytes and oligodendrocytes.
In August, Ward-Cherrier and his colleagues reported1 using human brain organoids of around 10,000 neurons to ‘recognize’ Braille letters. They first used a robot fitted with a tactile sensor to read the letters, and then converted the data collected for each letter into a distinct pattern of electrical pulses — varying the timing and intensity, for example — which they passed through a series of eight electrodes placed next to the surface of the organoid. These electrodes record the collective activity of many nearby neurons.
The researchers wanted to learn whether the patterns of firing in the organoid were different depending on the stimulation pattern it received, and whether those responses were consistent.
For each letter, they collected the response from each electrode, averaged them to yield an overall organoid output, and used machine learning to identify any patterns.
The results showed that, when fed electrical pulses corresponding to specific letters, a single organoid would produce the same characteristic response 61% of the time, on average. When responses from three organoids were combined, that went up to 83%. In other words, the organoids could perform a simple processing task: to distinguish between and identify inputs.
For Ward-Cherrier, it’s a solid proof of principle. “It is an initial kind of stab at showing that we can do these types of tasks. The next step is doing something a little bit more complex.” That could include interpreting messages from the cultured cells as instructions for the robot — to read the letter again, for example. Such abilities define what researchers call closed-loop-systems, which have not yet been demonstrated with human brain organoids — although a 2024 study2 reported that such a system, made of mouse neuronal organoids, could play Cartpole, a computer game in which the aim is to keep a wobbly pole upright on a moving cart.
Because the inputs and outputs in cultured systems are simple electrical signals, it’s easy to offer remote access to organoids through the web. So, even though the Braille-reading robot is based in Ward-Cherrier’s lab in Bristol, the organoids are grown and housed at the company FinalSpark, in Vevey, Switzerland.

Devices containing tiny clumps of human brain cells sit inside a fridge at FinalSpark in Vevey, Switzerland.Credit: Fabrice Coffrini/AFP via Getty
A self-confessed fan of science fiction, FinalSpark co-founder Fred Jordan says he wants to develop systems of biological neurons that can “perform some similar things as are done with AI today”.
There is a long way to go. As computers, the organoid systems are currently “totally useless from a practical perspective”, he admits. “There is a very big difference between dreaming or thinking about something and doing it for real. And I would love to be one of those people who make this step.”
Selected academic groups, such as Ward Cherrier’s, get free access to FinalSpark organoids, and many teams have signed up for this. A team at the University of Michigan in Ann Arbor, for example, is testing different types of stimulation to see how the organoids behave, and researchers at the Free University of Berlin are focusing on how machine-learning tools can best extract information from neural firing patterns.
For clients with deeper pockets, including private companies, monthly fees of US$5,000 can secure exclusive online access to an organoid system. And plenty do. “We have very, very big companies active in individual fields that you would think are disconnected to this,” Jordan says. Unlike with the free work done by academic groups, FinalSpark does not know what paying customers use the organoids for.
Other independent groups with organoid expertise are jumping into biocomputing. The neural organoids in Alysson Muotri’s lab at the University of California, San Diego, each contain about 2.5 million neurons of various types. Muotri aims to apply organoids to a real-world problem: predicting the path of possible oil spills in the Amazon jungle. The project, financed by an oil company, is due to conclude by 2028. “We’re taking the challenge,” he says. “We’ll see where we are in three years.”
Training ground
For many organoid users trying to run more-complex tasks, one immediate aim is to find ways to train the neurons and thereby encourage goal-directed behaviour. At present, the responses from FinalSpark’s lab-grown organoids have more in common with the reflex actions of the peripheral nervous system — when someone’s leg kicks in response to a tap below the knee, for example — than with the malleable processes that guide decision-making in the brain.
To handle more complexity, these neural systems must be able to learn. One way to encourage that, Jordan says, is to deliver neurotransmitters such as dopamine to try to tune the organoids’ responses to particular stimuli. Dopamine makes neurons more likely to fire and strengthens the synapses that connect them — two changes that make the same neural response to a stimulus more likely to be repeated in future.
Another is a technique called pattern training stimulation, which was used in 2022 by researchers at Cortical Labs, a company based in Melbourne, Australia, to encourage lab-grown brain cells to play the 1970s computer game Pong3.
Rather than working with organoids, they chose to create networks of cells in dishes (see ‘Computing with cells’). Then the researchers wired them up to a computer, which was programmed so that the response of the neurons to stimulation moved a virtual paddle while a virtual ball bounced around. To direct the paddle towards the ball, the researchers fed the neurons with an organized burst of electrical activity if the cells (at first, randomly) got it right. If the neurons moved the paddle in the wrong direction, they were blasted with chaotic white noise. Over time, the neurons learnt to hit the ball to receive the patterned response rather than the random one.
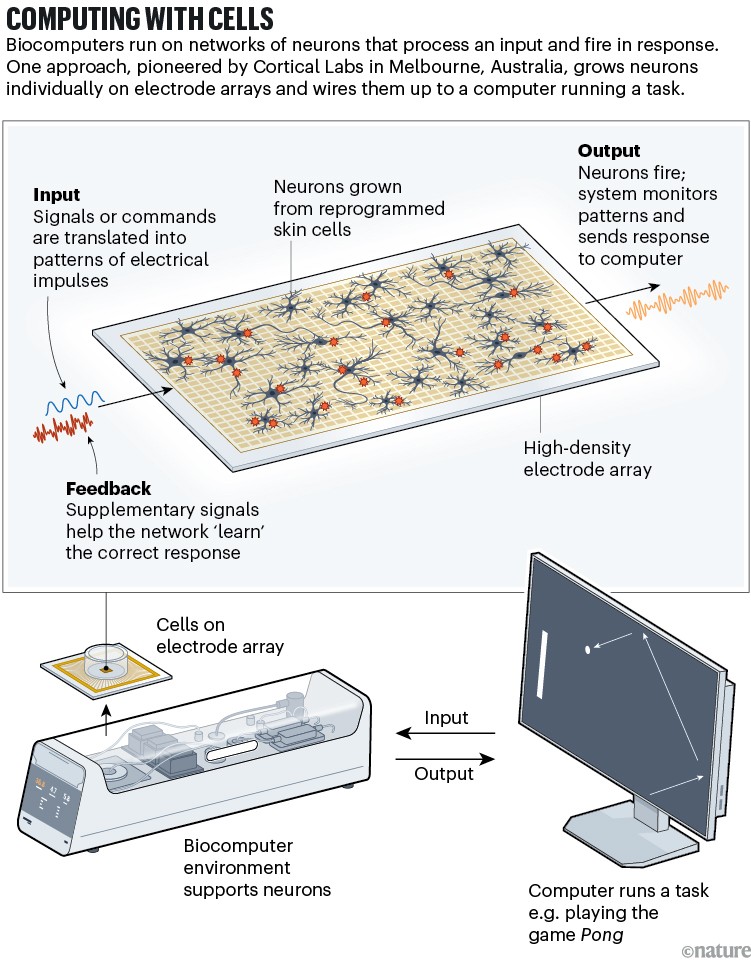
The strategy is drawn from the observation that brain cells tend to repeat activity that yields a predictable outcome, so will learn the kinds of behaviour that trigger familiar stimulation.
Cortical Labs has now developed a modular system that can link increasing numbers of individual wells, each containing at most 1,000 neurons, to form what the company calls a bioengineered intelligence. Each cell culture has a useful life of about six months before it must be replaced.