Mice
All animal procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Chinese Institute for Brain Research, Beijing (CIBR), and complied with the national guidelines for the housing and care of laboratory animals set by the Ministry of Health, China. Mice were housed in a specific pathogen-free facility on a 12-h light–dark cycle with ad libitum access to food and water. All experiments were conducted on male and female mice aged 8–16 weeks.
The study used WT C57BL/6J mice, Adora1−/− mice (NM-KO-225140, Shanghai Model Organisms Center), Adora2a−/− mice (NM-KO-200018, Shanghai Model Organisms Center), Nt5e−/− mice (provided by J. Chen, Wenzhou Medical University) and Rosa26-Cas9-GFP mice (Gt(ROSA)26Sortm1.1(CAG-cas9*, -EGFP) Fezh/J; The Jackson Laboratory, 024858). All mouse strains were subjected to CRS to induce depression, and WT, Nt5e–/–, Adora1−/− and Adora2a−/− mice were used for fibre photometry experiments.
Chemical reagents
Ketamine analogues were synthesized and provided by the Changchun Institute of Applied Chemistry, CAS, China (see below). Additional key chemicals were purchased from commercial sources, including norketamine hydrochloride (Tocris, 1970), (2R,6R)-HNK (Tocris, 6094), ticlopidine (Selleck, S0721), ketoconazole (Selleck, S1353), ritonavir (Selleck, S1185), dipyridamole (Selleck, S1895), LPS from Escherichia coli O127:B8 (LPS; Sigma, L3129), PSB36 (MCE, HY-103175), ZM241385 (Selleck, S8105), CHA (MCE, HY-18939), CGS21680 hydrochloride (MCE, HY-13201A), adenosine (MCE, HY-B0228), sodium [13C3]pyruvate (MCE, HY-W015913S), ADP (MCE, HY-W010918) and wheat germ agglutinin (Alexa Fluor 555; Thermo Scientific, W32464).
Compound synthesis and characterization
Full experimental procedures, compound characterization data (1H NMR and 13C NMR) and analytical spectra are provided in the Supplementary Information. A summary of the synthesis for the two key compounds (DCK and 2C-DCK) is presented below.
For the general procedure for the synthesis of 2-aryl-2-bromo-cycloketones, N-bromosuccinimide (1.5 equiv.) and dimethyl sulfoxide (2.0 equiv.) were added to a solution of 2-arylcyclohexan-1-one (1.0 equiv.) in CHCl3. The reaction mixture was stirred at room temperature and monitored by thin-layer chromatography. After completion, the reaction was quenched with saturated aqueous Na2S2O3 and water. The aqueous phase was extracted with CH2Cl2 (3 times). The combined organic layers were dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by flash column chromatography (petroleum ether/ethyl acetate) to afford the desired 2-aryl-2-bromo-cycloketone.
For the general procedure for the synthesis of ketamine derivatives (DCK and 2C-DCK), a solution of the appropriate 2-aryl-2-bromo-cycloketone (1.0 mmol) in anhydrous THF was cooled to the specified temperature (−25 °C) under a nitrogen atmosphere. The corresponding amine (methylamine for DCK; ethylamine for 2C-DCK; 4.0 equiv.) was added, and the reaction was stirred until thin-layer chromatography indicated complete consumption of the starting material. The reaction was quenched by the addition of saturated aqueous Na2CO3 and water. The mixture was extracted with CH2Cl2 (3 times), and the combined organic layers were dried over anhydrous Na2SO4. The solvent was removed in vacuo, and the residue was treated with diethyl ether and aqueous HCl. The aqueous layer was washed with diethyl ether, neutralized with saturated aqueous Na2CO3 and extracted with CH2Cl2 (3 times). The final organic layers were combined, dried over anhydrous Na2SO4 and concentrated to dryness under vacuum to produce the final product.
Below are the characterizations of DCK and 2C-DCK using NMR spectroscopy.
Compound 2 (DCK): 2-(Methylamino)−2-phenylcyclohexan-1-one
1H NMR (300 MHz, CDCl3): δ 7.45–7.34 (m, 2H), 7.33–7.19 (m, 3H), 3.01–2.79 (m, 1H), 2.48–2.19 (m, 3H), 2.03 (s, 3H), 2.01–1.91 (m, 1H), 1.90–1.61 (m, 4H). 13C NMR (75 MHz, CDCl3): δ 211.7, 138.9, 128.9, 127.6, 127.2, 70.0, 39.9, 35.5, 29.0, 27.9, 22.4.
Compound 3 (2C-DCK): 2-(ethylamino)−2-phenylcyclohexan-1-one
1H NMR (300 MHz, CDCl3) δ 7.42–7.33 (m, 2H), 7.31–7.20 (m, 3H), 2.90 (d, J = 11.0 Hz, 1H), 2.50–2.22 (m, 3H), 2.16 (s, 1H), 2.11–2.01 (m, 1H), 2.01–1.60 (m, 5H), 0.99 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ 211.5, 139.5, 128.9, 127.5, 127.1, 69.9, 39.8, 36.6, 36.1, 27.8, 22.4, 15.7.z.
AAV vectors
The following AAV vectors were produced in-house (M.L’s laboratory) by co-transfecting HEK293T cells with the following respective AAV plasmid and helper plasmids: AAV2/9-hSyn-GRABAdo1.0, AAV2/9-EF1a-DIO-GCaMP8s, AAV2/8-GfaABC1D-GRABATP1.0 and AAV2/8-GfaABC1D-cOpsin5-T2A-mCherry. Viral particles were purified by cesium chloride density gradient ultracentrifugation, dialysed into PBS and titred by quantitative PCR (qPCR) to 5–15 × 1012 viral genomes per ml51.
The following additional vectors were obtained: AAV-hSyn-GRABAdo1.0-mut was generated in Y.L’s Laboratory; AAV-EF1α-DIO-PercevalHR and AAV2/5-GfaABC1D-PercevalHR were produced in Z.W’s laboratory; and AAV2/8-GfaABC1D-mCherry was purchased from Taitool Bioscience.
For conditional knockout in the mPFC, sgRNAs targeting Adora1 (5′-GTGTAGCGGTAGCCAGCTGA-3′, 5′-CCGGAACTTGTGGATTCGGA-3′ and 5′-GATCAAGTGTGAGTTCGAGA-3′) and Adora2a (5′-TCGCCATCCGAATTCCACTC-3′, 5′-TCTGGCGGCGGCTGACATCG-3′ and 5′-AGCACACAAGCACGTTACCC-3′) were designed. Non-targeting control sgRNAs (5′-GCGAGGTATTCGGCTCCGCG-3′, 5′-GCTTTCACGGAGGTTCGACG-3′ and 5′-ATGTTGCAGTTCGGCTCGAT-3′) were also used. Each sgRNA, driven by a U6 promoter, was co-packaged with a CMV-driven saCas9-3×HA into either AAV2/9 (for neuronal targeting) or AAV2/8 (for astrocytic targeting) vectors.
Validation of global Adora1 and Adora2a knockout and mPFC-specific knockdown was performed using qPCR on cDNA from brain tissues with the following primer pairs: Adora1 knockout (F: 5′-TGTGCCCGGAAATGTACTGG-3′, R: 5′-TCTGTGGCCCAAATGTTGATAAG-3′); Adora2a knockout (F: 5′-GTGCTGTCATTCGCCATCGG-3′, R: 5′-GGGAGCAACACAAAAGCGAAG-3′); Adora1 sgRNA for conditional knockdown (F: 5′-GCCAGAAACCCAGCATCCTC-3′, R: 5′-CAGAAAGGTGACCCGGAACT-3′); and Adora2a sgRNA (F: 5′-GCCATCCCATTCGCCATCA-3′, R: 5′-GCAATAGCCAAGAGGCTGAAGA-3′). All primers were designed to span critical exon junctions or CRISPR–Cas9-targeted regions, with reaction specificity confirmed by melt curve analysis and agarose gel electrophoresis. qPCR was carried out in triplicate using SYBR Green chemistry under standard cycling conditions.
Surgical procedures
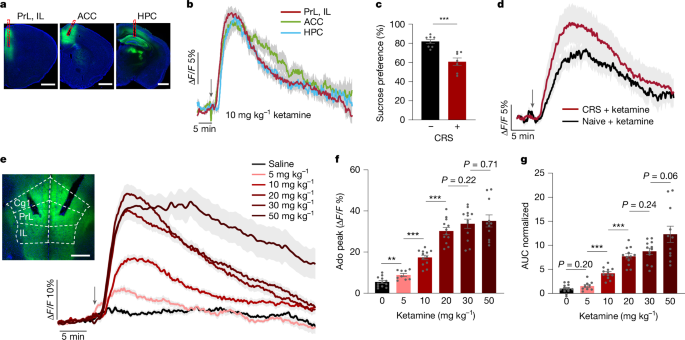
Mice were anaesthetized with avertin (250 mg kg−1, i.p. injection) and secured in a stereotaxic apparatus (RWD). Following skull exposure, a small craniotomy was made above the target region. AAV injections were performed using a microsyringe pump (Nanoliter 2010 Injector, WPI) at a rate of 46 nl min–1 via a glass pipette. Injection coordinates (anterior–posterior (AP), medial–lateral (ML), dorsal–ventral (DV) in mm from bregma) were as follows: PrL and IL in the mPFC (+1.78, ±0.88, −2.15, respectively) with a 15° lateral-to-medial angle; ACC (+1.15, ±0.70, −1.47, respectively) with a 15° lateral-to-medial angle; HPC (−2.54, ±2.00, −1.60, respectively) and NAc (+1.20, ±1.20, −4.65, respectively).
For fibre photometry and optogenetic stimulation experiments52,53, optical fibre implantation was performed after viral injection. Optical fibres (FT200UMT, Thorlabs) mounted in ceramic ferrules were positioned above the mPFC, ACC, HPC or NAc, with the tip located 0.1 mm above the injection site. For intracranial adenosine injection, a cannula (62004, RWD) was implanted into the lateral ventricle (AP: −0.45, ML: −1.84, DV: −2.45 DV) at a 15° lateral-to-medial angle.
For in vivo two-photon imaging, mice were imaged 14 days after viral injection to allow time for virus expression. Subsequently, under anaesthesia, a 3-mm diameter skull aperture was drilled at the injection site and covered with a glass window. A stainless-steel head-restraining bar integrated with an imaging chamber was affixed with dental cement. Mice recovered for 1 week before imaging, which was conducted in the awake state.
Fibre photometry
In vivo fluorescence signals from GRABAdo1.0, GRABAdo1.0-mut, GRABATP1.0 and GCamp8s signals were recorded using a multichannel fibre photometry system54,55 (ThinkerTech). A 470-nm blue LED provided sensor excitation, with the intensity adjusted to minimize photobleaching. The resulting emission was passed through a dichroic mirror (MD498, Thorlabs) and a bandpass filter (525 ± 19.5 nm; MF525–39, Thorlabs) before detection.
Fibre photometry of PercevalHR signals was conducted on a separate two-colour multichannel optical fibre photometry system (Optical Imaging Facility, CIBR). To measure the intracellular ATP/ADP ratio, the PercevalHR sensor was alternately excited at 405 nm and 470 nm, with emission collected at 525 nm, as previously described37,56. The ratio of fluorescence intensity from 470 nm excitation to that from 405 nm excitation (F470/F405) was calculated to represent changes in the ATP/ADP ratio.
For pharmacological studies, mice implanted with an optical fibre were habituated for 15–20 min in a behaviour chamber (20 × 20 × 35 cm) to establish a baseline signal. Following habituation, the mice given an i.p. injection of the drug of interest, and fluorescence signals were continuously recorded. Animal behaviour was monitored using a top-mounted camera.
For acute hypoxia experiments, mice were placed in a cylindrical chamber with ports for the optical fibre, gas flow and an oxygen sensor (ST8100A, Smart Sensor). After a 20-min acclimation period with a continuous flow of air (21% O2), hypoxia was induced by mixing the room air with 100% N2 via a three-way valve. GRABAdo1.0 and GRABAdo1.0-mut signals were continuously recorded during acclimation, hypoxia and subsequent reoxygenation with air. After the trial, the chamber was cleaned with 70% ethanol and dried.
Fibre photometry data were analysed using custom Matlab scripts. Fluorescence changes were calculated as ΔF/F = (F – F0)/F0, where F0 represents the mean fluorescence during a baseline period before drug administration. For recordings longer than 30 min, photobleaching was corrected by subtracting a ‘blank’ signal, which was recorded from the same animals on a separate day without any drug or saline administration. From the resulting ΔF/F traces, the following parameters were quantified: peak amplitude, the maximum signal intensity reached after stimulation or drug administration; time to peak, the latency from administration to the peak amplitude; onset time, the time for the signal to reach 20% of the peak amplitude; rise time, the interval during which the signal increased from 20% to 90% of its peak; and decay time, the time taken for the signal to decrease to 50% of its peak amplitude.
In vivo two-photon imaging
During imaging sessions, mice remained awake and were gently restrained using a custom-built head-fixation device. Images were acquired 100–150 µm below the dura mater using a Stellaris 8 Dive multiphoton microscope (Leica, ×25 water-immersion lens with NA 1.05). The microscope was calibrated for consistent illumination and exposure settings across all imaging sessions. A 20-min baseline was recorded before ketamine (10 mg kg−1, i.p. injection) or an equivalent volume of saline as a control was given. Following the injection, imaging continued for an additional 20 min to capture changes in neural activity.
To correct for lateral shifts in two-photon images, we used the Image Stabilizer plugin in ImageJ (Fiji, v.2.14.0). For time trace analyses of fluorescence signals based on the region of interest, the Time Series Analyzer V3 plugin in ImageJ was used. ΔF/F values were calculated using customized Matlab scripts (MathWorks), and heatmaps or time traces were generated accordingly. Statistical significance was assessed using Prism 9 (GraphPad Software). All data are reported as the mean ± s.e.m. in the figures.
Confocal imaging of GRABAdo1.0 in cultured cells
HEK293T cells were cultured on 35-mm poly-d-lysine-coated glass-bottom dishes (NEST, 801002). At 60–70% confluency, cells were transfected with the GRABAdo1.0 plasmid using Neofect DNA transfection reagent (Neofect Biotech). Imaging was performed 48 h after transfection on a Zeiss LSM 880 inverted confocal microscope (Carl Zeiss) using a ×20/0.8 NA Plan-Apochromat objective. The GRABAdo1.0 sensor was excited with a 488-nm argon laser.
Behavioural assays
All behavioural assays were performed on animals 12–16 weeks old. Most behavioural assays were performed during the light phase, except for the SPT, which was performed during the dark phase to maximize the consumption of solution. Behavioural analyses were performed blinded to experimental conditions.
CRS assay
Mice were subjected to an environmental acclimation period of 3 days preceding initiation of the experiment. Subsequently, the mice were immobilized utilizing custom-fabricated restraining tubes (50 ml centrifuge tubes) with ventilation apertures to ensure the maintenance of normal respiration. The restraint protocol was implemented for a duration ranging from 4 to 6 h per day over a consecutive 14-day period57. After completion of the modelling phase, a behavioural experiment was conducted for the purpose of assessment.
LPS-induced inflammatory depression model
The LPS-induced depression model is a well-established paradigm for rapidly inducing depressive-like behaviours in mice32. In brief, i.p. administration of a low dose of LPS (0.83 mg kg–1, E. coli O127:B8, Sigma-Aldrich) induces a mild inflammatory response and triggers transient sickness behaviour within 24 h. Subsequently, between 24 and 72 h after injection, mice develop persistent depression-like phenotypes, including anhedonia, behavioural despair and anxiety-like responses.
FST assay
Mice were individually placed into Plexiglass cylinders (26.5 cm high × 18 cm in diameter) containing 14 cm of water maintained at 25 ± 1 °C. The test lasted for 6 min under standard illumination, with a digital video camera recording from the side. Immobility time during the final 4 min of the test, defined as the period during which mice floated passively with only minimal movements necessary to maintain balance, was scored by a trained observer blinded to the experimental treatments58.
SPT assay
Mice were habituated to two bottles of drinking water in their home cages for 2 days, followed by exposure to two bottles containing 2% sucrose solution for an additional 2 days. After habituation, mice were deprived of water for 24 h and then presented with one bottle of 1% sucrose solution and one bottle of water for 2 h during the dark phase. The positions of the bottles were switched after 1 h to control for side preference. Sucrose preference was calculated as the percentage of sucrose intake relative to the total fluid intake (sucrose and water combined)59.
Open-field test
Locomotor activity was assessed using an infrared open-field system (Med Associates; 50 × 50 × 30 cm). Baseline activity was measured in WT, Adora1–/ and Adora2a–/– mice during a 10-min session. Mice were placed at the centre of the arena, and total travel distance was recorded using an automated tracking system. To evaluate the effects of ketamine and DCK, mice were acclimated for 15 min in the arena, followed by drug administration. Locomotor activity was monitored for 75 min after treatment, with total travel distance recorded over the 90-min session. In a separate experiment, WT, Adora1–/– and Adora2a–/– mice underwent the same acclimation period (15 min) followed by ketamine and saline administration, with activity recorded for 30 min after treatment.
ECT in mice
Following CRS, mice were anaesthetized with avertin, and their ears were cleaned with 70% ethanol. ECT was delivered via ear-clip electrodes using a YC-3 Bipolar Programmable Stimulator, applying an electrical current of 40 mA (100 Hz, 10-s duration, 0.5-ms pulse width)60. This stimulation induced a tonic–clonic seizure lasting approximately 10 s. Sham-treated animals underwent identical handling, including electrode attachment, but no current was delivered.
aIH treatment
Mice subjected to CRS were exposed to aIH using an interval conditioning regimen42. The regimen comprised five cycles of a 5-min hypoxia period at 9% O2, each followed by a 5-min normoxic interval (21% O2), repeated daily for three consecutive days. The oxygen concentration was monitored in real time using an integrated sensor (ST8100A, Smart Sensor). The antidepressant effects of aIH were assessed using established models of depressive-like behaviour, including the FST and SPT. For fibre photometry recordings of adenosine dynamics, a 15-min baseline recording was performed before aIH exposure to monitor signal changes in response to the interval training protocol.
Local drug infusion
Bilateral 26-gauge guide cannulae (RWD Life Science) were stereotaxically implanted to target the mPFC (AP: +1.78 mm, ML: ±0.5 mm, DV: −2.05 mm from bregma) and the HPC (AP: −2.54 mm, ML: ±2.00 mm, DV: −1.60 mm). Following a 7-day postoperative recovery period, during which dummy cannulae maintained patency, mice were subjected to a 2-week CRS paradigm. For microinfusions, adenosine (0.1 µg µl–1) were dissolved in sterile 0.9% saline. Solutions were delivered bilaterally (1 µl per side) at a rate of 0.2 µl min–1 via 33-gauge injectors connected to a microsyringe pump. The injectors remained in place for 7 min after infusion to allow for diffusion. Behavioural testing, using either the FST or SPT, was conducted 24 h after ketamine administration. At the conclusion of all experiments, cannula placement was histologically verified following the infusion of wheat germ agglutinin conjugated to Alexa Fluor 555 (WGA-555; 1 µl per side; Thermo Fisher Scientific).
Optogenetics
WT and Nt5e–/– mice previously subjected to CRS were used for optogenetic experiments. Animals were injected with AAVs expressing either AAV-GfaABC1D-cOpn5-T2A-mCherry (cOpn5 group) or AAV–GfaABC1D-mCherry (control group) in the target brain region. For stimulation, blue light (473 nm; MBL-III-473, Changchun New Industries Optoelectronics) was delivered through the implanted optical fibre at 20 Hz for 10 min (peak power at fibre tip: 0.75 mW)61. Stimulation timing was controlled by a Master-8 pulse generator (AMPI). FSTs and SPTs were performed 1 h after the cessation of stimulation to assess antidepressant-like effects.
LC–MS quantification of drug concentrations
WT mice were administered either adenosine receptor agonists or ketamine derivatives. At specified time points after injection (10 min for ketamine derivatives; 30 min or 24 h for agonists), mice were deeply anaesthetized with isoflurane. Whole blood was collected via retro-orbital bleeding, and mice were subsequently transcardially perfused with ice-cold PBS. Brain tissue was rapidly dissected, weighed and flash-frozen in liquid nitrogen. Blood samples were allowed to clot at room temperature and then centrifuged at 3,500g for 10 min at 4 °C to separate the serum. For analysis, serum proteins were precipitated by adding four volumes of acetonitrile to one volume of serum, followed by vortexing and centrifugation. Brain tissue was homogenized in 80% acetonitrile using a bead-based homogenizer, and the resulting lysate was clarified by centrifugation. Supernatants from both serum and brain preparations were diluted 100-fold with 80% acetonitrile. Drug concentrations were then quantified using a SCIEX 7500 triple quadrupole mass spectrometer.
Mitochondrial metabolic flux analysis
Mitochondrial isolation
Mitochondria were isolated from the prefrontal cortex of adult mice using a commercial kit (EpiZyme, PC205) with minor modifications. In brief, dissected tissue was homogenized, and crude mitochondria were pelleted by differential centrifugation. Highly purified mitochondria were then obtained by density gradient centrifugation according to the manufacturer’s protocol, washed and resuspended for downstream assays.
Metabolic modulation and metabolite extraction
Purified mitochondria were incubated for 30 min at 37 °C in an intracellular buffer containing sodium [13C3]pyruvate and ADP, with or without ketamine at various concentrations. Following incubation, mitochondria were pelleted, and metabolites were extracted using cold (–40 °C) 50% aqueous methanol solution followed by phase separation with chloroform. The resulting aqueous phase, containing polar metabolites, was collected for analysis.
LC–MS-based metabolomics
Metabolite profiling was performed using hydrophilic interaction chromatography (HILIC) on an XBridge BEH Amide column (Waters) coupled to a Q Exactive Plus Orbitrap mass spectrometer (Thermo Fisher Scientific). Samples were separated using a gradient of aqueous ammonium acetate/hydroxide (pH 9.4) and acetonitrile. Mass spectrometry was operated in negative ion mode at a resolution of 140,000 (m/z 200), with an AGC target of 1 × 106 and a scan range of m/z 75–1,000. Metabolite identification, quantification and isotopic tracing were conducted using El-MAVEN software, with correction for natural isotope abundance. We performed PCA of the metabolomic data and defined ellipse 95% confidence intervals based on the multivariate t-distribution (Fig. 3h). The corresponding source data and analysis code are provided in the source files.
Ex vivo electrophysiology
Mice (C57BL/6J mice, 7–8 weeks old) were anaesthetized with an overdose of avertin and transcardially perfused with ice-cold, oxygenated choline-based slicing solution (in mM: 110 choline chloride, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 1.3 NaH2PO4, 25 NaHCO3, 10 glucose, 1.3 sodium ascorbate and 0.6 sodium pyruvate). Coronal brain slices (200 µm) containing the mPFC were prepared using a vibratome (Leica VT1200). Slices were first recovered at 34 °C for 40 min in oxygenated artificial cerebrospinal fluid (ACSF; in mM: 125 NaCl, 2.5 KCl, 2 CaCl2, 1.3 MgCl2, 1.3 NaH2PO4, 1.3 sodium ascorbate, 0.6 sodium pyruvate, 10 glucose and 25 NaHCO3) and then maintained at room temperature for at least 1 h before recording.
Whole-cell patch-clamp recordings were performed in Mg2+-free ACSF to relieve the voltage-dependent block of NMDARs. Recording pipettes (4–6 MΩ) were filled with a caesium-based internal solution (in mM: 115 CsMeSO3, 20 CsCl, 10 HEPES, 2.5 MgCl2, 4 sodium ATP, 0.4 sodium GTP, 10 sodium phosphocreatine, 0.6 EGTA and 5 QX-314; pH 7.25–7.30). To isolate NMDAR-mediated excitatory postsynaptic currents (eEPSCs), recordings were performed in the presence of NBQX (10 µM, MCE) and picrotoxin (100 µM, Tocris). eEPSCs were evoked by local stimulation (0.4-ms pulse, every 20 s) while holding the cell at −65 mV. Following a 4-min stable baseline, various concentrations of test compounds were bath-applied for 16 min. The degree of blockade was quantified as the eEPSC amplitude during the final minute (15–16 min) of drug application, normalized to the baseline.
Western blotting
The mPFC total protein for BDNF and CD73 detection were performed in WT, Adora1−/−, Adora2a−/− and Nt5e−/− mice. Animals were anaesthetized with isoflurane, and the mPFC tissue was quickly dissected from the brain and homogenized in lysis RIPA buffer (50 mM Tris HCl, pH 7.4 (Sigma), 150 mM NaCl, 1% Triton X-100 (Sigma) and protease inhibitor cocktail (Sigma)) on ice. After determining the protein concentration with the bicinchoninic acid assay, 35 mg of total protein from each mPFC sample was loaded onto a 4–20% SDS–PAGE gel for separation. Proteins were then transferred to a polyvinylidene fluoride membrane for western blot analyses. Rabbit anti-BDNF (1:1,000; Abcam, ab108319), rabbit anti-CD73 (1:1,000; Cell Signaling Technology, 13160), rabbit anti-GAPDH (1:5,000; Cell Signaling Technology, 2118), rabbit anti-HSP90 (1:1,000; Cell Signaling Technology, 4874) and HRP-conjugated antibody goat anti-rabbit IgG (1:30,000; Sigma-Aldrich, AP156P) were used, along with high-sensitivity ECL reagent (Perkin Elmer). All bands were analysed using ImageJ software.
Histology and immunohistochemistry
For tissue preparation, mice were anaesthetized with an overdose of pentobarbital and intracardially perfused with PBS followed by 4% paraformaldehyde in PBS. Brains were postfixed in 4% paraformaldehyde for 4 h at room temperature or overnight at 4 °C, then cryoprotected in 30% sucrose until they sank. Coronal sections (35 µm) were prepared using a cryostat (Leica CM1950). Virus expression and fibre implantation sites were verified in brain sections using an Olympus VS120 slide scanner with a ×10 objective.
Statistics and reproducibility
All experiments were independently performed ≥3 times with mice randomly assigned to each group, and investigators were blinded to allocation during behavioural experiments and outcome assessment. No statistical methods were used to predetermine the sample sizes.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.