We adopted a five-step pipeline to identify candidates for enhancing neural repair with a streamlined path that could lead to human testing (Fig. 1a). Step 1 involves characterizing the transcriptomic state or ‘signature’ of a cell type that exhibits a beneficial biological effect. In this case, we used the transcriptome of corticospinal motor neurons that are capable of regeneration after SCI. We reported previously that, within 2 weeks of SCI, the corticospinal neuron undergoes transient transcriptional reversion to an embryonic state—a state in which it is capable of regenerating1. These data are publicly available. In Step 2, we perform an in silico screen to compare the regenerating corticospinal transcriptome with shifts in the transcriptome generated by more than 1,300 small molecules or compounds in the Broad CMap (https://bioconductor.org/packages/release/data/experiment/html/ConnectivityMap.html). Drugs and small molecules in CMap have been exposed to various cell lines in vitro, and effects of these compounds on the transcriptome of the cultured cells have been characterized3. Thus, we searched CMap for compounds that generated transcriptomic shifts that parallel the state of regenerating corticospinal neurons. In Step 3, we took the lead candidates from the in silico screen and applied them to a medium-throughput in vitro assay consisting of cultures of adult motor cortex neurons; this screen became available only recently2 and provides what could be the most useful in vitro system for predicting potential in vivo effects in the mature nervous system, because the screen consists of adult rather than embryonic or postnatal neurons. To date, in vitro cellular screens of the nervous system consisted of either embryonic or early postnatal neuronal cultures, which may not accurately reflect responses of adult central nervous system (CNS) neurons. Immortalized neural cell lines4 suffer from similar limitations. Although adult dorsal root ganglia (DRG) neurons can be cultured routinely, these cells exhibit fundamentally different transcriptomes before and after injury1,5,6 and, again, might not be accurate tools for predicting therapeutic benefits of candidate therapies for injured brain and spinal cord motor neurons. Induced pluripotent stem cells or neurons induced from other cell types also often exhibit early cellular markers7. Cultures of adult neurons therefore may represent the most optimal in vitro screen developed for clinical translation2. Step 4 consists of in vivo validation of the top drug arising from the in vitro screen in a model of SCI. Finally, Step 5 consists of validating candidate compounds in cultures of adult primate cortical neurons, including human adult cortex. This step follows rather than precedes in vivo testing because of the scarcity of the primate and human neuronal resource: normal human neurons in particular are available only occasionally from surgical specimens and in our case were obtained from an individual undergoing tumour resection through a cortical window, where pathology confirmed the cortical biopsy was normal tissue.
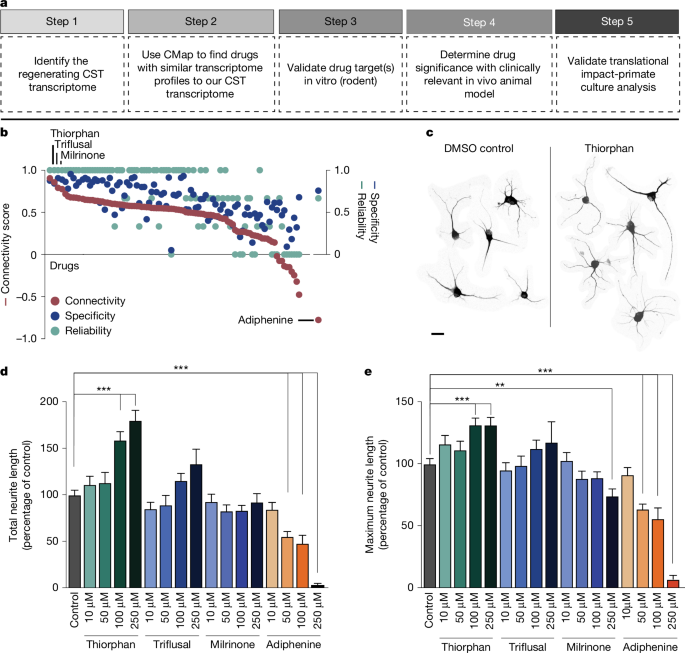
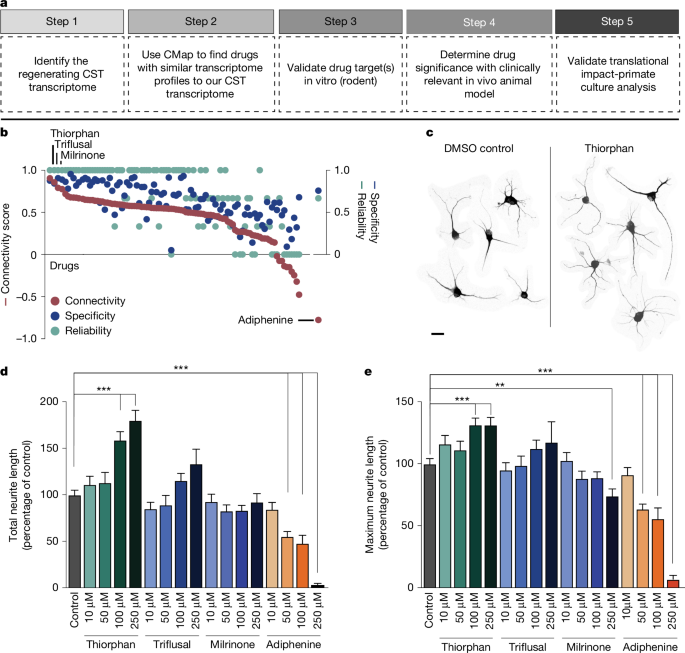
a, Drug discovery pipeline consisting of five steps: (1) creation of a transcriptomic dataset; (2) in silico analysis using CMap; (3) medium-throughput in vitro screen; (4) in vivo testing of lead candidate(s); and (5) in vitro monkey and human validation. CST, corticospinal tract. b, In silico analysis shows ranking of compounds in CMap on the basis of connectivity score (left y axis), specificity score (blue, right y axis) and reliability score (green, right y axis) (see text for further detail). c, Neurite extension in composite image of cortical neurons treated with DMSO control and thiorphan (250 μM), Tuj1 labelling. d,e, In vitro screen of top three ‘hits’ from in silico screen, together with predicted negative modulator, adiphenine. Tested in cultures of adult mouse primary cortical neurons for 5 days in vitro, three independent biological replicates were performed on separate days, each using neurons from four adult mice. For each condition, data from all neurons measured across replicates were combined to yield n = 200 neurons per condition. Each neuron was spatially separated and analysed as an independent observation. Total neurite outgrowth per cell (Tuj1 labelling) is shown for each condition. Median values: DMSO (82.9); thiorphan (90.7, 99.8, 132.4, 166.1); triflusal (74.3, 61.7, 94.5, 124.5); milrinone (78.9, 77.4, 68.5, 75.5); adiphenine (74.4, 44.5, 31.7, 3) (d). Maximum neurite length per cell is shown for each condition. Median values: DMSO (93.3); thiorphan (111.1, 105.4, 122.1, 128); triflusal (84.4, 85, 104.9, 114.3); milrinone (100.6, 81.7, 79, 78.6); adiphenine (87.4, 60, 30.6, 6.23) (e). Statistical significance was determined by two-tailed Student’s t-test (**P < 0.01, ***P < 0.001). Error bars ± s.e.m. (d,e). Scale bar, 25 μm (c).
Characterizing the transcriptomic state
Step 1 was accomplished in this study by using our previous data describing the transcriptome of the regenerating corticospinal neuron1, as referenced above.
In silico analysis
In silico analysis directly followed the original methodology of Lamb et al.3, identifying several compounds that most closely matched the expression profile of the regenerating corticospinal system (Fig. 1b and Supplementary Fig. 1a). A ‘connectivity score’ with a value between +1 and −1 described the similarity between the corticospinal transcriptomic profile and an individual compound’s transcriptomic profile, where +1 was most similar and −1 was least similar to the corticospinal dataset (Fig. 1b). We further calculated a ‘reliability score’ for each compound that takes into account the connectivity score, P value, false detection rate (FDR), ‘instance count’ (number of experiments that include the compound), non-null score (the ratio of experiments that have a consistent score based on most experiments) and ‘specificity score’ derived from the distribution of enrichment scores for gene lists from MSigDB; see Methods for a more detailed description of the reliability score. The top compounds that met the criteria of highest enrichment, reliability and specificity scores were considered ‘hits’ in this assay and included: quinostatin (highest enrichment score +0.91 ± 0.07), thiorphan (+0.86 ± 0.11), triflusal (+0.79 ± 0.13) and milrinone (+0.78 ± 0.18), whereas adiphenine was the most negatively correlated (−0.78 ± 0.18; Fig. 1b and Supplementary Fig. 1b). Biological annotation (Methods) indicated that quinostatin inhibits PI3 kinase; thiorphan is a neutral endopeptidase inhibitor; triflusal inhibits nuclear factor κB, phosphodiesterase and COX1, and milrinone is a phosphodiesterase inhibitor (Supplementary Fig. 1a). Quinostatin was not available for purchase and attempts at synthesis did not yield adequate quality compound. Thiorphan has been characterized primarily as a neutral endopeptidase inhibitor targeting neprilysin8. It has also been linked to neuroprotective properties in models of perinatal excitotoxic brain lesions9 and diabetic retinopathy10, and has β-amyloid degrading properties11. Thiorphan, milrinone and triflusal were taken forward as positive candidates, and adiphenine was used as a negative control.
In vitro adult motor cortical culture screen
We assessed the lead candidate compounds in dissociated cultures of the adult (postnatal day 60) mouse motor cortex2. An average of 10,000 neurons per single motor cortex were obtained, and we quantified total neurite outgrowth and maximal neurite length per cell 5 days after exposure to the candidate compounds. Several drug doses were applied (Fig. 1d,e), including the predicted negative modifier of corticospinal growth, adiphenine. Significant overall differences in neurite outgrowth were present using various compounds (drug type × dose interaction generalized estimating equation for total neurite outgrowth, Wald chi-square = 60.67, P < 0.0001; drug type × dose interaction generalized estimating equation for total maximum neurite length, Wald chi-square = 227.78, P < 0.0001). Thiorphan stood out as most effective both in measures of total neurite outgrowth and neurite length: at a dose of 250 μM it increased total neurite outgrowth by 80% compared with controls (post hoc generalized estimating equation, Wald chi-square = 29.69, P < 0.001; Fig. 1d,e) and longest neurite length by 30% (post hoc generalized estimating equation, Wald chi-square = 12.62, P < 0.001; Fig. 1d,e). The effect of thiorphan was dose dependent, with peak effects occurring at doses between 100 μM and 250 μM. The effect of the next candidate drug for enhancing corticospinal regeneration, triflusal, was more modest, with an increase in neurite outgrowth of 30% at its peak dose of 250 μM with no significant change in longest neurite length; none of these changes reached statistical significance (Fig. 1e). The third candidate in rank order, milrinone, did not significantly influence either total neurite outgrowth or longest neurite length at any dose (Fig. 1d,e). Validating the in silico screening process, the predicted negative regulator of corticospinal growth, adiphenine, strongly and significantly reduced total neurite outgrowth and longest neurite length in a dose-dependent manner (post hoc generalized estimating equation, P < 0.001; Fig. 1c–e).
In vivo testing in a model of SCI
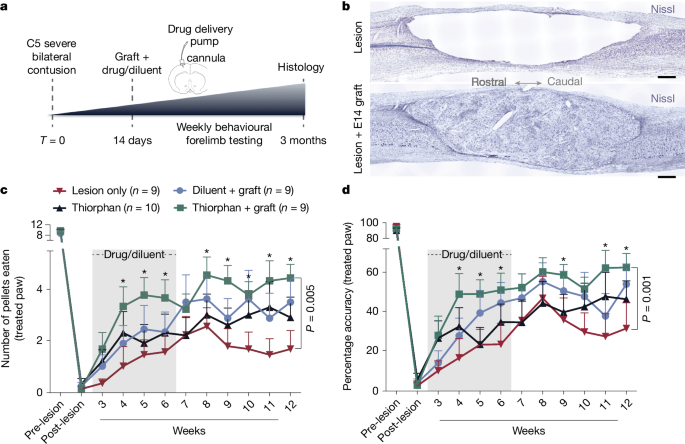
Given the success of thiorphan in demonstrating efficacy in the in vitro screen, it advanced to Step 4: in vivo testing in a clinically relevant model of SCI. Adult Fischer 344 rats underwent severe contusive bilateral cervical SCI at the C5 spinal cord segment; mid-cervical lesions are the most common levels of human injury12 (Supplementary Fig. 2a,b). We then waited 2 weeks—a clinically relevant time to delay intervention to allow subject recovery from the acute effects of injury (Fig. 2a). Animals were then divided into one of four treatment groups: (1) lesion alone (n = 9), (2) thiorphan alone (n = 10), (3) diluent + NPC graft (n = 9) and (4) thiorphan + NPC graft (n = 9). We tested diluent + NPC grafts as a positive control in this lesion model because these cells support axonal regeneration into the lesion site, the formation of new neural relays across the injury site and partial functional recovery13,14. We also compared thiorphan alone with NPCs. The combination of thiorphan + NPCs aimed to determine whether effects of both treatments were additive, thereby representing a method of further augmenting recovery after severe cervical SCI. Thiorphan was infused continuously into the left motor cortex because it does not cross the blood–brain barrier. Infusions continued for 4 weeks starting 2 weeks after injury at a dose of 100 mM (0.25 μl h−1), based on extrapolation of in vitro dosing to in vivo dosing15. An in vivo neprilysin cleavage assay confirmed the bioactivity of intraparenchymal motor cortex infusions of thiorphan over 1, 2 and 4 weeks (Supplementary Fig. 2c). Spinal cord NPC grafts were obtained from Fischer 344 embryonic-day-14 (E14) spinal cords as reported previously16 and injected through the dura into the lesion cavity 2 weeks post SCI (Fig. 2b; Methods). Two weeks before euthanasia, the corticospinal projection was traced anterogradely using an AAV9-CAG–Ruby2sm_Flag.
a, In vivo experimental outline of a clinically relevant SCI model. b, Nissl stain of a severe C5 bilateral contusion model. Sagittal section, rostral to left. Top, large contusion cavity in an ungrafted animal after 3 months. Bottom, NPC graft filling the lesion cavity and providing substrate for potential corticospinal axon regeneration. c, Skilled forelimb successful pellet retrieval with right paw on Montoya staircase23 ± s.e.m. Animals that received thiorphan and a substrate for axonal regeneration into the lesion site—an NPC graft—exhibit significant functional recovery over time compared with lesioned controls (P = 0.005, group × time interaction, Poisson generalized linear model). Treatment with thiorphan alone also trended towards significance (P = 0.14). The lesion model applied in this experiment is the most severe that we have tested, and NPC grafts also exhibited a trend towards better outcomes than lesioned controls but this did not reach statistical significance. Grey shading, period of thiorphan infusion into cortex. d, Thiorphan + NPC graft group also exhibits significant recovery of pellet retrieval accuracy in this severe lesion model (P = 0.001). Other treated groups trend towards improved outcomes compared with lesioned controls. Accuracy represents the number of pellets eaten divided by the number of pellets displaced plus the number of pellets eaten. *P < 0.05 (c,d). Scale bar, 500 μm.
Twelve weeks after SCI, animals treated with thiorphan + NPC grafts exhibited a significant, twofold improvement in forelimb grasping success (number of pellets grasped and eaten) after severe mid-cervical SCI compared with lesioned controls (Poisson generalized linear model, Wald chi-square = 7.92, P = 0.005; Fig. 2c). Recipients of thiorphan alone or NPC grafts alone exhibited lesser degrees of forelimb recovery compared with lesioned controls that did not reach statistical significance (Poisson generalized linear model, Wald chi-square = 2.16, P = 0.14 comparing thiorphan with lesion alone, and Wald chi-square = 2.07, P = 0.15 comparing grafts-only with lesion alone; Fig. 2c). The accuracy of pellet retrieval (percentage of pellets grasped that are eaten) also improved significantly in animals that received thiorphan + NPC grafts (Gamma generalized linear model, Wald chi-square = 10.13, P = 0.001) compared with lesioned controls (60% accuracy versus 30% accuracy; Fig. 2d). Thus, as suggested by in silico and subsequent in vitro screens, thiorphan significantly improves functional outcomes when combined with neural stem cell grafts to sites of SCI by enhancing the potency of NPC grafting.
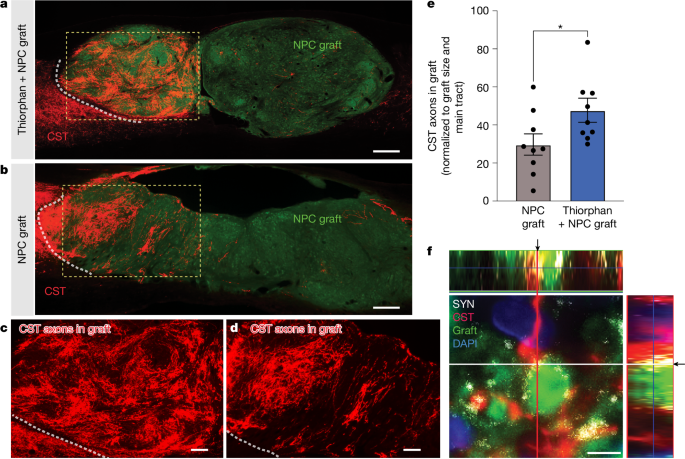
To examine anatomical mechanisms underlying beneficial effects of thiorphan infusion on functional outcomes, we assessed corticospinal axon regeneration into stem cell grafts occupying the lesion site. Previously, we have shown that corticospinal axons regenerate into NPC grafts placed into sites of SCI and form synapses. Further, grafted NPC neurons extend axons from the lesion site to host neurons below the lesion and form synapses; consequently, new neural relays are formed across the lesion that support functional improvement13,16,17,18,19. In the present experiment, thiorphan significantly increased corticospinal regeneration into grafts by 60% (P < 0.05; Fig. 3a,c,e) compared with individuals that received NPC grafts alone (Fig. 3b,d,e and Supplementary Fig. 3a,b). Thiorphan administration in the absence of NPC grafts could not promote corticospinal regeneration into the lesion cavity because there was no cellular substrate onto which injured corticospinal axons could extend. Thin plane confocal microscopy showed colocalization of corticospinal axon terminals with synaptophysin apposed onto grafted neurons, indicating putative synapse formation from regenerating host axons to grafted neurons (Fig. 3f). Nearly all grafted animals in this experiment exhibited complete graft filling of the lesion cavity (Supplementary Fig. 4a,b). Cortical infusion of thiorphan over 4 weeks caused no damage or neuronal loss in the motor cortex (Supplementary Fig. 4c,d).
a, CST axons labelled anterogradely with Flag (red) show greater regeneration into E14 neural progenitor stem cell grafts (green (green fluorescent protein (GFP))) in animals with thiorphan cortical infusions. The white dotted line indicates the host–graft interface; the boxed region is shown at higher magnification in c. b, Fewer corticospinal axons regenerate into animals that did not receive thiorphan cortical infusions. The white dotted line indicates the host–graft interface; the boxed region is shown at higher magnification in d. c,d, Higher magnification views of the boxed regions in a (c) and b (d) showing regenerating corticospinal axons. e, Corticospinal regeneration into graft occupying lesion site is increased 1.6-fold in the presence of thiorphan infusion into the motor cortex (*P < 0.05, two-tailed t-test). In the absence of a graft, no host axons are present in the lesion site; non-grafted animals could not be quantified. f, Regenerating host corticospinal axons form putative synapses with grafted neurons, based on colocalization of regenerating CST (red) with synaptophysin (SYN, white) apposed to grafted neuron (GFP, green). DAPI in blue. Arrows indicate the exact orthogonal view intersection point, single plane. Scale bars, 500 μm (a,b); 200 μm (c,d); 5 μm (f).
We also examined whether thiorphan treatment influenced corticospinal axonal sprouting above the lesion site: there were no significant differences in corticospinal axon density among the four animal groups (Supplementary Fig. 5a,b). Although thiorphan was administered into the motor cortex, we also examined whether there was a difference in the growth of serotonergic or sensory (calcitonin gene-related-peptide-labelled) axons into the graft; as expected, there was no difference between thiorphan + NPC graft and the diluent + NPC grafted groups (Supplementary Fig. 6a–f). We further examined whether thiorphan administration into the cortex influenced the host–graft glial border (glial fibrillary acidic protein immunoreactivity); it did not (Supplementary Fig. 7a–c). Finally, we examined whether thiorphan administration into the cortex affected graft differentiation: it did not (Supplementary Fig. 7d–k); as expected, neither did it affect graft-derived axon extension into the host (Supplementary Fig. 7l,m). Thus, beneficial effects of thiorphan administration were detectable on the corticospinal system to which it was targeted.
Testing in primate systems
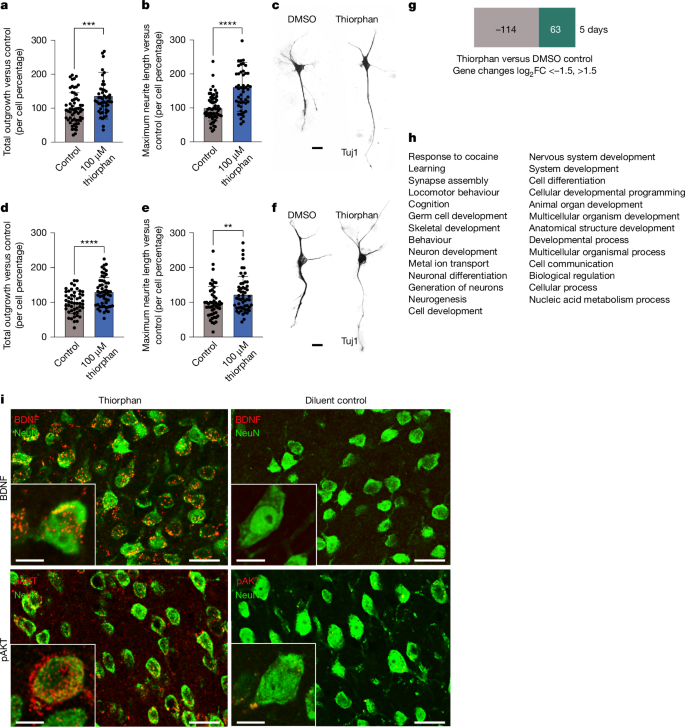
The final step in this process, Step 5, extended the clinical relevance of this work by determining whether thiorphan can also promote growth from adult primate motor cortex and human cortical neurons (Fig. 4a–c). This step is performed last because these samples—particularly normal human cortex samples—are highly valuable. Human participant approval was obtained for these procedures. Neurons were isolated from the monkey M1 motor cortex, dissociated and cultured, adapted from ref. 2, and treated with 100 μM thiorphan for 5 days in vitro (two replicates). These cultures are free of NPCs when assessed by markers such as Nestin and Sox2. Thiorphan treatment significantly increased total neurite outgrowth by 36% (P < 0.001) and maximum neurite length by 61% (P < 0.0001) compared with control conditions (Fig. 4a–c). We then proceeded to culture the human cortex: normal cortex from the middle temporal gyrus of a 56-year-old man was obtained from biopsy, the tissue was dissociated and neuronal cultures established (adapted from ref. 2; Supplementary Fig. 8). Treatment with 100 μM thiorphan for 5 days in vitro (Fig. 4d–f) resulted in a significant increase in total neurite outgrowth by 30.3% ± 7.3% (P < 0.0001) compared with control conditions. Thiorphan also exhibited a significant increase in longest neurite length by 23.0% ± 9.4% (P = 0.016) compared with controls. Thus, thiorphan retains biological activity when tested in monkey and human cortical cultures, exhibiting roughly 40% of the potency exhibited in rats in effects on total neurite outgrowth and 70% of the potency exhibited in rats in terms of longest neurite length.
a, Thiorphan significantly increased total neurite outgrowth from primary cultures of adult cynomolgus macaque motor cortex labelled for Tuj (***P < 0.001, two-tailed t-test). Control cultures received DMSO. Each point represents the total neurite length of individual neurons. Samples were run as technical duplicates, with neurons cultured for 5 days. b, Thiorphan treatment also significantly increased the maximum neurite length per cell by 1.8-fold (****P < 0.0001, two-tailed t-test). c, Representative images of cultures of adult primate motor cortex treated with diluent or thiorphan. d, Thiorphan significantly increased total neurite outgrowth from primary cultures of normal 56-year-old human cortex neurons labelled for Tuj (****P < 0.0001, two-tailed t-test). Neurons cultured for 5 days. Each point represents the total neurite length of one neuron. e, Thiorphan treatment of human cortical neurons also significantly increased the maximum neurite length per cell (**P < 0.01, two-tailed t-test). f, Representative images of 56-year-old human cortical neurons in culture labelled for Tuj1. g, RNA sequencing was performed on cultures of adult primary motor cortex from rhesus monkeys treated for 5 days with thiorphan or vehicle (DMSO). A total of 177 genes changed ±1.5-fold on a log2 scale. h, Gene ontology of these 177 genes demonstrates clear enrichment for developmental processes (Gene Ontology database24, https://doi.org/10.5281/zenodo.10536401). i, To identify in vivo mechanisms recruited by thiorphan treatment, rat brains were removed after 2 weeks of thiorphan infusion and compared with diluent-infused controls. Thiorphan treatment increased immunolabelling for both BDNF and phospho-AKT in the infused motor cortex, reflecting a shift to a state that is developmental and pro-regenerative. Scale bars, 20 μm (c,f). Scale bars, 25 μm, inset scale bars, 10 μm.
Thiorphan was identified as a potential candidate for improving axonal growth after CNS injury by virtue of its ability to shift the cellular transcriptome to an embryonic, pro-growth state. To assess whether this mechanism was actually recruited in neuronal target cells, we performed RNA sequencing (RNA-seq) of macaque M1 motor cortex cells exposed to thiorphan for 5 days in vitro compared with control cultures lacking thiorphan exposure (Fig. 4g). Because we had one sample of thiorphan-treated and one sample of vehicle (dimethylsulfoxide (DMSO))-treated cells, we compared mRNA species changing ± 1.5 log2 units between the two samples: 177 genes reached this cut-off threshold (Supplementary Fig. 1c). Gene ontology of these 177 genes identified 29 biological processes and remarkably, 16 of these 29 were related to development, including neuron development, nervous system development, and synapse assembly (Fig. 4h). To further examine mechanisms associated with thiorphan delivery, we infused it intracortically for two weeks in rats and performed immunolabelling of the infused region compared with animals that received vehicle infusions: thiorphan administration resulted in increased expression of brain-derived neurotrophic factor (BDNF) (Fig. 4i) and phospho-AKT (Fig. 4i) in the infused region, paralleling higher levels of these molecules observed both in neural development and successful regeneration. These findings support a mechanistic framework in which thiorphan modifies the developmental, metabolic and signalling state of cells to impact neuronal regeneration, consistent with its initial identification on the basis of its ability to replicate the effects of SCI in driving neurons developmentally backward towards a state of embryonic development.