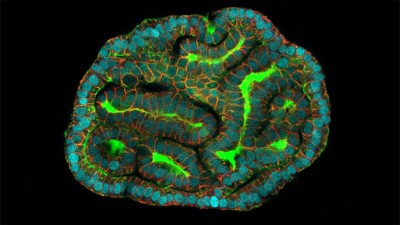
Collections of human cells generated from different sources can be used to test new drugs. Credit: Lewis Houghton/SPL
When it comes to evaluating drugs or understanding basic biology, researchers are increasingly favouring various innovative experimental approaches over animal models or immortalized human cell lines.
Such approaches, known as NAMs — short for ‘novel alternative methods’ or ‘new approach methodologies’ — remove the ethical problems associated with experimenting on animals. By being able to capture much more human genetic variability than the animal models and cell lines traditionally used in research can, they also promise to identify safe and effective treatments more reliably and at a fraction of the cost.
Examples of NAMs include: multicellular in vitro systems that mimic the biological and mechanical properties of human organs and tissues; chemical (in chemico) assessments of the interactions between biological molecules outside a cell or organism; and computational (in silico) modelling approaches, such as those that predict how molecules will interact.
Why simply ending animal testing isn’t the answer in biomedical research
Some governments and regulators worldwide are now pushing for the increased use of NAMs in research, partly because of pressure from animal-rights groups. In April, for example, the US Food and Drug Administration (FDA) announced plans to phase out its requirement for animal testing in the development of monoclonal antibody therapies, among other drugs. And just last month, the US National Institutes of Health (NIH) awarded US$87 million to set up the Standardized Organoid Modeling Center, a national resource dedicated to the development of organoid-based NAMs “to reduce reliance on animal modeling”.
Yet, despite all of these developments, early adopters of NAMs — ourselves included — are still encountering resistance. Although there has been some progress (see ‘On the rise’), many such researchers struggle to publish their work, particularly in high-impact journals. Some continue to be asked by reviewers to validate their studies in animals or, when applying for grants, find that funders view pilot data more favourably if those data include animal studies. Early-career researchers, in particular, report feeling pressured into including experiments on animals, even when there seems little scientific justification for doing so.
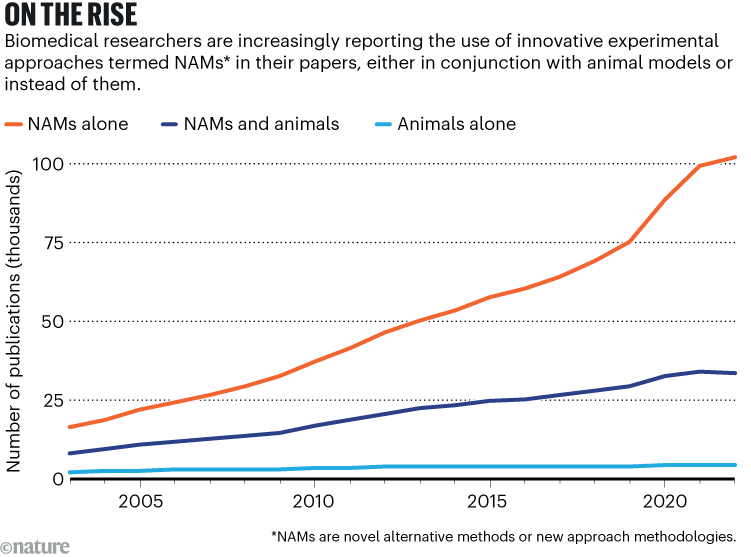
Source: K. Taylor et al. Front. Lab Chip Technol. 3, 1426895 (2024)
To take advantage of the opportunities that NAMs now present — and of the growing number of initiatives aimed at boosting the development and use of these approaches — changes are needed in how scientific journals, regulators and funders operate. This would help to ensure that those who are implementing these transformative technologies are applauded, not penalized.
What is a NAM?
Some cell- and tissue-based NAMs involve human induced pluripotent stem (iPS) cells. These can be generated in the laboratory from somatic cells, such as blood or skin cells. They continuously self-renew and, with the appropriate chemicals and mix of growth factors, can differentiate into almost any cell type in the body. Thus, using blood draws or biopsies, researchers can, in principle, obtain an unlimited supply of cells representing any organ type, patient or patient population.
Organoids and spheroids are among the NAMs that can be generated from iPS cells or other cells. These 3D structures allow researchers to analyse cell behaviour, model diseases and test drugs in in vitro systems that mimic the architecture, function and cell types of a human organ, or the conditions provided by natural tissues.
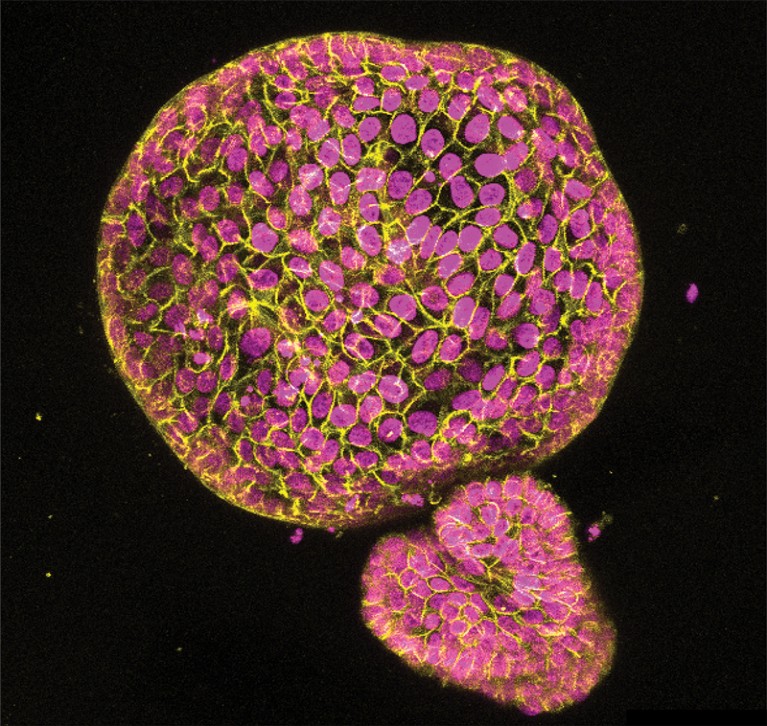
An organoid made from cells found in the lining of the uterus. Credit: Konstantina Nikolakopoulou, Turco Lab, Friedrich Miescher Institute for Biomedical Research
Using collagen, other similar proteins or polymer hydrogels that are free from animal derivatives, models are now being designed that biologically and mechanically mimic the microenvironments of tumours and the extracellular matrix (the network of proteins and other molecules that surrounds cells and tissues).
Tumour spheroids generated from a person’s tumour tissue, or an entire biopsy, can be implanted into an extracellular matrix. And a circulatory system involving a pump can even push nutrients through the spheroids1. These technologies allow researchers to control and monitor the tumour microenvironment much more efficiently than if they were using animal models or patient-derived xenografts, a technique in which a biopsy of an individual’s tumour is implanted into a mouse model.
Modelling of the blood–brain barrier has also gained momentum. Using brain organoids, researchers can investigate whether certain drugs — or even cancer cells — can cross this barrier.
Meanwhile, advances in NAMs in chemico and in silico are helping to accelerate drug development by flagging promising candidates for testing.
The FDA’s AnimalGAN models, for example, use artificial intelligence to generate animal-based data in toxicology that would otherwise need to be obtained using animal studies. Likewise, mathematical approaches, such as quantitative structure–activity relationship models, can work out the mathematical relationship between the chemical structure of a compound and its biological activity and predict the properties of new chemical entities.
Trials in a dish
Globally, on average 86% of drug candidates fail in human clinical trials2. This is either because of problems with toxicity or, more commonly, because the drugs are not sufficiently effective in humans3. This inefficiency seems to be mainly the result of experiments in animal models failing to predict human responses to medications4.
For the past half a century, drug development has involved testing compounds in animals for years before exposing them to any human biology. By bringing human biology to the earliest phases of drug discovery, NAMs could significantly reduce the time and money needed to obtain positive results.
Organoids grown from amniotic fluid could shed light on rare diseases
Using ‘clinical trials in a dish’ (CTIDs) — in which each ‘dish’ might consist of a panel of 100 organoids generated using stem cells or diseased tissues from 100 individuals, say — researchers can test therapies on a representative sample of patients before they test them in animals or in conventional clinical trials5.
CTIDs could be especially useful for developing therapies to treat rare diseases caused by genetic mutations. More than 100 mutations of the LMNA gene, for example, are known to cause rare inherited heart diseases6. Using CTIDs, drug developers could tailor therapies to individuals with different mutations by simultaneously testing the effectiveness of several drugs on multiple patients’ cells in vitro7,8.
Besides benefiting the discovery and development of drugs for inherited diseases, CTIDs could provide crucial training data for AI models that have been designed to aid drug discovery9.
NAMs could also help to reduce health inequalities. At scale, almost all of the alternative models cost less than animal work does, which could reduce costs for patients10. And with the use of human iPS cells and patient-derived tissue in vitro, it should be possible to perform more-inclusive clinical trials in which medication is tested in under-represented populations, including those from low- and middle-income countries.
Shifting mindsets
As early users of NAMs, we’ve been encouraged by various initiatives, both in the United States and Europe, to reduce the use of animal testing in research.
The FDA’s April announcement about monoclonal antibody therapies is part of a broader effort to ‘replace, reduce and refine’ the use of animals in research11. A few weeks afterwards, the NIH declared that it would establish the Office of Research Innovation, Validation, and Application (ORIVA), in part to coordinate efforts to develop and expand the use of NAMs.