Mouse models
Young adult (8- to 12-week-old) wild-type C57BL/6NCrSlc mice obtained from the Japan SLC, Fos2A-iCreERT2 (TRAP228, JAX 030323) mice obtained from the Jackson Laboratory, Adra1a flox mice49,74 obtained from RIKEN (RBRC11837) and Adrb1 flox mice50 (accession number CDB1068K, generated by RIKEN Center for Biosystems Dynamics Research) were used for in vivo experiments. TRAP2 mice were bred and maintained in our animal facility, backcrossed with C57BL/6NCrSlc at least eight times before use for experiments. Wild-type C57BL/6NCrSlc female mice were obtained from the Japan SLC to obtain postnatal day 0–1 (P0–P1) pups. Experiments were done using both male and female mice. Number of mice used in each experiment is listed in the main text and/or figure legends accordingly. Mice were housed in groups of 2–5 in a temperature- and humidity-controlled room (23 ± 3 °C, 45 ± 5% humidity) under a 12-h light:dark cycle (lights on from 07:00 to 19:00) and given ad libitum access to water and laboratory mouse diet at all times. After fibre implantation, the mice were single housed under a 12-h light:dark cycle (lights on from 07:00 to 19:00) and given ad libitum access to water and laboratory mouse diet at all times. After fibre implantation, the mice were singly housed. All experiments were approved by the RIKEN Animal Care and Use Committee.
Cell lines
AAVpro 293T cells (Clontech, 632273) were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, 11995073) with fetal bovine serum (FBS; Cosmo Bio, CCP-FBS-BR-500) and 0.1% antibiotic-antimycotic (Gibco, 15240062). Cells were grown in a humidified cell culture incubation with 95% air, 5% CO2 at 37 °C.
Viral vector construction
All plasmid constructs were generated using standard molecular biology techniques and the In-Fusion HD Cloning Kit (Clontech). The pZac2.1 GfaABC1D-Rpl22-HA plasmids (Addgene plasmid #111811) were digested with XhoI and NotI, which removed the Rpl22-HA sequence. Multiple cloning sequences (MCS) comprised of restriction enzyme sites that were absent from the backbone pZac2.1 plasmid were generated via gene synthesis (Eurofins Genomics) and incorporated to produce pZac2.1-GfaABC1D-MCS plasmids. The PCR-amplified DIO-GFP sequence from Addgene plasmid #28304 was cloned into the mammalian expression vector pCAG-tdTomato (Addgene #83029) between EcoRI sites to generate pCAG-DIO-EGFP-tdTomato. The PCR-amplified DIO-EGFP-tdTomato was inserted into the XbaI and NotI sites of the pZac2.1-GfaABC1D-MCS plasmid to generate pZac2.1-GfaBC1D-DIO-EGFP-tdTomato plasmids. The PCR-amplified WPRE sequence from pFBAAV-CAG-XCaMP-R-WPRE (a gift from H. Bito12) was inserted into the NotI site of the resulting plasmid to generate pZac2.1-GfaABC1D-DIO-EGFP-tdTomato-WPRE plasmids. The plasmids were digested with NotI and AgeI, which removed the tdTomato sequence, and a PCR-amplified mScarlet-I sequence (Addgene #98816) was incorporated, and the resulting plasmids were digested with XhoI and KpnI, which removed the EGFP sequence, and the mNG sequence from Addgene #99135 was ligated using DNA ligation kit (Takara) to generate pZac2.1-GfaABC1D-DIO-mNG-mScarlet-I-WPRE plasmids. The mScarlet-I sequence was removed with restriction enzymatic digestion with PspOMI and NotI to generate pZac2.1-GfaABC1D-DIO-mNG-WPRE plasmids. The PCR-amplified hM4Di-mCherry sequence from Addgene #92286 was cloned into the PmeI site of pAAV-RAM-d2TTA::TRE-MCS-WPRE (Addgene #63931) to generate pAAV-RAM-d2TTA::TRE-hM4Di-mCherry-WPRE plasmids. pAAV-GfaABC1D-Flag-mAdrb1-WPRE, pAAV-GfaABC1D-mGrm3-mCherry-WPRE, pAAV-GfaABC1D-Lck-mCherry-iβARK(D110A), pAAV-GfaABC1D-Lck-mCherry-iβARK, pAAV-GfaABC1D-DIO-Lck-mCherry-iβARK(D110A), pAAV-GfaABC1D-DIO-Lck-mCherry-iβARK pAAV-Fos-FlpoERT2 and pAAV-GfaABC1D-FRT-mNG plasmids were designed by the authors and constructed by VectorBuilder. The PCR-amplified DIO-Lck-mCherry-iβARK(D110A) and DIO-Lck-mcherry-iβARK sequence from pAAV-GfaABC1D-DIO-Lck-mCherry-iβARK(D110A) and pAAV-GfaABC1D-DIO-Lck-mCherry-iβARK was clone into the EcoRI site of pAAV-GfaABC1D-MCS-4x6T-WPRE (Addgene #196417) to generate pAAV-GfaABC1D-DIO-Lck-mCherry-iβARK(D110A)-4x6T and pAAV-GfaABC1D-DIO-Lck-mCherry-iβARK-4x6T. pAAV-GfaABC1D-mGrm3-mCherry-WPRE was digested with EcoNI and AccI to remove mGrm3-mCherry, and PCR-amplified mGrm3 was cloned back to the backbone to generate AAV-GfaABC1D-mGrm3-WPRE plasmids. The PCR-amplified XCaMP-Gf-WPRE from the pFBAAV-CAG-XCaMP-Gf-WPRE plasmid (a gift from H. Bito75) was cloned into the NcoI and HindIII site of pAAV-hSyn1-cyto-mRuby3-iATPSnFR1.0 (Addgene #102557) to generate pAAV-hSyn1-XCaMP-Gf-WPRE plasmids. The mCherry-CAAX sequence digested with EcoRI of the pZac2.1-GfaABC1D-mCherry-CAAX plasmid (a gift from T. Takano76) was ligated into the EcoRI and EcoRV the resultant plasmids to generate pAAV-hSyn1-mCherry-CAAX-WPRE plasmids. All constructs were sequenced before use. All our constructs have been deposited to Addgene in the Nagai Lab repository for unrestricted distribution (http://www.addgene.org/Jun_Nagai, Supplementary Table 4).
Viral production
Recombinant AAVs were generated by triple transfection of AAVpro 293T cells (Clontech, 632273) using polyethylenimine (PEI; Polysciences inc., 24765-100). Cells were cultured up to 50–70% confluency in DMEM (Gibco, 11995073) with FBS (Cosmo Bio, CCP-FBS-BR-500) and 0.1% antibiotic-antimycotic (Gibco, 15240062). The plasmids and PEI were mixed with Opti-MEM (31985-088) and kept for 5 min at room temperature. The mixture was combined with a DNA mix which contained pAAV Capsids, pHelper (Agilent Technologies, 240071-54) and pAAV transgenes. All these plasmids had a concentration of 4.2 μg per dish. The DNA mix was then applied with PEI/Opti-MEM, and incubated for 20 min at room temperature. Then, this solution was added to the cells and incubated for 24 h. After incubation, the medium was changed to DMEM with 2% FBS and 0.1% antibiotic-antimycotic. Viral particles were collected from the medium at 120 h post-transfection. Cell pellets were centrifuged (3,500 rpm, 30 min, 4 °C). The supernatant was filtered by Setricup (Merc, S2HVU02RE) and pellets were resuspended in FBS-free DMEM and freeze-thawed three times. In the case of PHP.eB capsid virus production, the supernatant was resuspended in DMEM and fractured by harshly pipetting. Thawed pellets were centrifuged (3,500 rpm, 10 min, 4 °C) again and the supernatant was filtered by Setricup (Merc, S2HVU02RE). Media containing viral particles were centrifuged (22,000 rpm, 2 h, 4 °C) by Optima XE-100 (Beckman Coulter, A94516). The pellets were collected with 10–50 μl PBS and clarified by centrifugation (4,000 rpm, 10 min, 4 °C). The supernatant-containing viral particles were stored at 4 °C. AAV titre was analysed by quantitative PCR (QuantStudio 12 K; Applied Biosystems) using primers designed to selectively bind AAV2 inverted terminal repeats (forward: 5′-AACATGCTACGCAGAGAGGGAGTGG-3′, reverse: 5′-CATGAGACAAGGAACCCCTAGTGATGGAG-3′).
Viral injections
All surgical procedures were conducted under general anaesthesia using continuous isoflurane (induction at 5%, maintenance at 1.5% to 2% vol/vol). Depth of anaesthesia was monitored continuously and adjusted when necessary. Intravenous administration of AAV-PHP.eB (50 µl to deliver 2.5 × 1012 genome copies (GCs) per mouse) was performed by injection into the retro-orbital sinus of TRAP2 mice. Two weeks after AAV-PHP.eB GfaABC1D-DIO-mNG-WPRE or AAV-PHP.eBGfaABC1D-DIO-mNG-mScaret-I-WPRE injections, mice were administered 4-OHT for inducing Cre recombination under the Fos promoter (Fos tagging) and then returned to their home cage until they were euthanized 7 days after tagging. In some cases, Fos tagging was performed using the FLP–FRT system (fDIO), and mice were euthanized one day after tagging. For stereotaxic microinjection of AAVs, mice were fitted into a stereotaxic frame (David Kopf Instruments) with their heads secured by blunt ear bars and their noses placed into a veterinary grade anaesthesia and ventilation system (VetEquip 911103). The surgical incision site was then cleaned with 70% ethanol (vol/vol). Craniotomies (1–2 mm in diameter) were made powered by a high-speed drill at specific coordinates based on the bregma position. Bilateral viral injections were conducted using a stereotaxic apparatus (David Kopf Instruments) to guide the placement of beveled glass pipettes (World Precision Instruments, 1B100-4) into the regions of interest: the basolateral amygdala (anterior–posterior (AP): −1.48 mm, medial–lateral (ML): 3.3 mm, dorsal–ventral (DV): 5.00 mm to bregma) and LC (AP: −5.4 mm, ML: 0.85 mm, DV: 3.7 mm to bregma). Total of 0.3 μl of AAV was delivered at 0.1 μl min−1 using a syringe pump (SmartTouch Pump, World Precision Instruments). The glass pipette was withdrawn 5–10 min after the infusion to prevent the backflow. Mice were then sutured, returned to their home cage, and allowed to rest for at least two to three weeks before any assessments were conducted. AAV titres (GCs per ml) were adjusted with sterile saline to deliver the indicated genome copies into the regions of interest: AAV2/5-GfaABC1D-DIO-mNG-WPRE (3.0 × 1012), AAV2/5-GfaABC1D-hM3Dq-mCherry (3.0 × 1012), AAV2/DJ8-RAM-d2TTA::TRE-hM4Di-WPRE (3.0 × 1013), AAV2/5-GfaABC1D-Flag-mAdrb1-WPRE (3.0 × 1012), AAV2/9-rTH-Cre (3.0 × 1012), AAV2/8-hSyn1-DIO-hM3Dq (1.0 × 1013), AAV2/8-hSyn1-DIO-hM4Di (1.0 × 1013), AAV2/8-hSyn1-DIO-mCherry (3.0 × 1012), AAV2/DJ8-Ef1a-hM3Dq (4.8 × 1011), AAV2/DJ8-Ef1a-hM4Di (4.0 × 1012), AAV2/5-hSyn1-mCherry-CAXX (3.0 × 1012) AAV2/9-hSyn1-GRABNE2h-WPRE (2.7 × 1012), AAV2/1 GfaABC1D-cAMPinG1-NE (1.3 × 1013), and AAV2/1 GfaABC1D-RCaMP3 (1.5 × 1013), GfaABC1D-GCaMP6f (1.1 × 1013), AAV2/5-GfaABC1D-iβARK-mCherry (1.3 × 1012), AAV2/5-GfaABC1D-iβARK(D110A)-mCherry (1.3 × 1012), AAV2/5-GfaABC1D-Lck-mCherry-iβARK (1.3 × 1012), AAV2/5-GfaABC1D-Lck-mCherry-iβARK(D110A) (1.1 × 1013), AAV2/5-GfaABC1D-DIO-Lck-mCherry-iβARK-4x6T (3.0 × 1013), AAV2/5-GfaABC1D-Lck-mCherry-iβARK(D110A) (3.0 × 1013), AAV2/5-Fos-FlpoERT2 (3.0 × 1012), AAV2/5-GfaABC1D-fDIO-mNG (1.0 × 1012), AAV2/5-GfaABC1D-Cre-4x6T (1.2 × 1013).
Drug administration in vivo
4-OHT (Sigma, H6278) was dissolved at 25 mg ml−1 in 100% ethanol with agitation at 37 °C for 15 min and was then aliquoted and stored at −20 °C for up to several weeks until use. Before use, 4-OHT was redissolved in ethanol by shaking at 37 °C for 15 min and corn oil (Sigma, 259853 and S5007) was added to give a final concentration of 3 mg ml−1 4-OHT. The ethanol was evaporated by incubation at 50 °C. The final 3 mg ml−1 4-OHT solutions were always used on the day they were prepared. All 4-OHT injections were delivered intraperitoneally at 50 mg kg−1 2–4 weeks after AAV injection into TRAP2 mice for inducing Cre recombination under the Fos promoter.
CNO (Tocris, 4936) was administered intraperitoneally 0.5 h before the initiation of behavioural assessments. CNO was administered intraperitoneally 20 min before 4-OHT intraperitoneal injection. Propranolol (MedChemExpress, HY-B0573) was dissolved in dH2O at 100 mM and stored at −20 °C. The final solutions were always used on the day they were prepared.
Mice were placed on doxycycline (40 mg kg−1, Bio-Serv, F4159) chow 24 h prior to and for at least 1 week after viral injection, switched to doxycycline-free chow 48 h before recall, and euthanized 24 h later. IGFBP2 antibody was infused bilaterally through implanted guide cannulas targeting basolateral amygdala. Infusions were performed using an internal cannula connected to a 10-µl Hamiltion syringe. One microgram of IGFBP2 antibody (1 mg ml−1, 1 µl per site) was infused into mice immediately after a fear recall paradigm at 400 nl min−1 using a syringe pump (SmartTouch Pump, World Precision Instruments).
Immunohistochemical analysis
Mice were deeply anaesthetized with isoflurane and transcardially perfused with PBS, followed by 4% paraformaldehyde (PFA) in PBS for fixation. The dissected brains were post-fixed overnight at 4 °C in 4% PFA in PBS, and cryoprotected in 30% sucrose in PBS. The brains were embedded with OCT compound (Sakura Finetek Japan, 4583) and sliced into coronal sections with a thickness of 50 μm at −20 °C using cryostat (CryoStar NX70, Thermo Fisher Scientific, 956960). The sections were washed with PBS 3 times for 5 min each and stored in 50% glycerol (Fujifilm, 075-00616) in PBS at −20 °C. For staining with c-Fos antibody, the sections were permeabilized with PBS containing 1% Triton X-100 (Nacalai tesque, 35501), incubated at room temperature for 3 h, and then incubated with 0.3% Triton X-100 in PBS containing 1% normal donkey serum (NDS, Sigma-Aldrich, D9663) at room temperature for 1 h for blocking, and then incubated at 4 °C overnight in blocking buffer. With the following primary antibodies, Triton X-100 was used at 0.1% for permeabilization and PBS containing 1% NDS was used for blocking and antibody reactions: rat anti-GFAP (1:500, Thermo Fisher Scientific, 13-0300), mouse anti-S100β (1:500, Sigma-Aldrich, S2532), goat-Sox9 (1:100, R&D systems, AF3075) mouse anti-RFP (1:500, MBL, M155-3), rabbit anti-RFP (1:500, Rockland, 600-401-379), mouse anti-NET (1:2,000, MAb technologies, NET05-2) and rabbit anti-c-Fos (1:5,000, Synaptic Systems, 226003). After washing with PBS 3 times for 10 min each, the sections were incubated at room temperature for 2 h in blocking buffer with the following secondary antibodies: Alexa Fluor 488 donkey anti-rat, Alexa Fluor 488 donkey anti-chicken, Alexa Fluor 568 donkey anti-mouse, Alexa Fluor 568 donkey anti-rabbit, Alexa Fluor 568 donkey anti-goat, Alexa Fluor 647 donkey anti-rabbit, Alexa Fluor 647 donkey anti-mouse, Alexa Fluor 647 donkey anti-chicken (1:1,000, Abcam, ThermoFisher and Jackson ImmunoResearch). The sections were incubated at 4 °C overnight in blocking buffer with secondary antibodies when staining with anti-c-Fos antibody. After washing with PBS 3 times for 10 min each, the sections were mounted on slides (Fluoromount-G, Southern bio, 0100-35) and visualized under a confocal laser microscope (Olympus, FV3000) and a fluorescent microscope (Olympus, VS200). Immunoreactivities were analysed using ImageJ software (NIH). The numbers of c-Fos+ neurons (Fig. 5o and Extended Data Fig. 8b) and astrocytes (Extended Data Fig. 4b) were quantified based on a consistent c-Fos intensity threshold for each experiment. This threshold was defined such that the number of c-Fos+ neurons or astrocytes corresponded to the values previously reported18,42,77. Number of cells, field of views, sections and mice used in each analysis is listed in the main text and/or figure legends accordingly.
Visual stimulation
Mice were singly housed in a light-proof chamber for 48 h. On the day of tagging, mice were exposed to 1 h of light inside the chamber and injected intraperitoneally with 50 mg kg−1 4-OHT either 24 h, 12 h, 6 h or 3 h before or 0 h, 3 h, 6 h, 12 h or 24 h after the termination of light exposure. Mice were returned to the dark chamber for an additional two days and then returned to a regular light-dark cycle until the time of euthanasia seven days after tagging.
Contextual fear conditioning
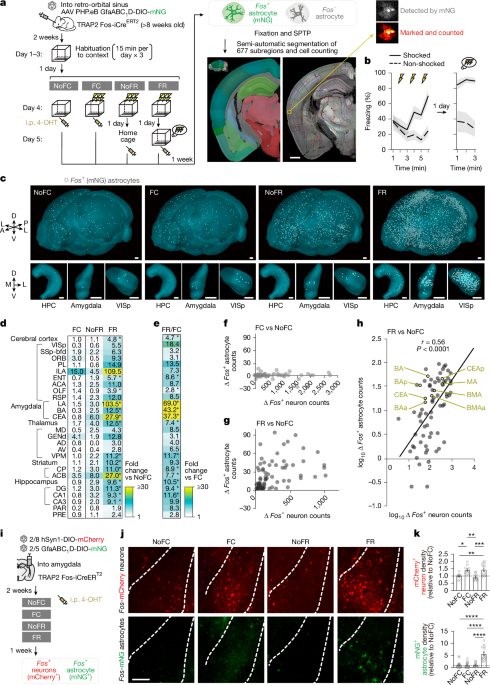
Behavioural tests were performed during the light cycle between 07:00 and 19:00. Temperature and humidity of the experimental rooms were maintained at 23 ± 2 °C and 55 ± 5%, respectively. Background noise (55 dB) was generated by a white noise generator (Ohara). All the experimental mice were transferred to the behaviour testing room at least 1 h before the tests to acclimatize to the environment and to reduce stress. Mice were habituated by freely exploring the conditioning chamber (CtxA) that consisted of a square partition (30 × 30 × 15 cm; 100 lux and no odour) with a grid floor wired to a shock generator (Ohara) for 15 min per day for 3 days. On the fourth day, they were either fear conditioned (1 time or 3 times shock; 2 s, 0.3 mA) (FC group) or presented with no shocks (NoFC group) in the conditioning chamber. One day after the conditioning, mice were either returned to CtxA for 3 min for fear memory recall (FR group) or kept in their home cages (NoFR group). Freezing durations exceeding 70% in the conditioned chamber 1 day post-conditioning with 3 foot shocks or exceeding 50% 1 day post-conditioning with a single foot shock, are considered indicative of sufficient conditioning. All the experimental mice received intraperitoneal injections of 4-OHT (50 mg kg−1) immediately after the task for Fos tagging. Freezing was automatically measured throughout the testing trial by the TimeFZ4 software (version 2021_7_29, Ohara) and subsequently processed by a blinded investigator. The chambers were cleaned with 70% ethanol between tests. In some cases, 10 mg kg−1 Propranolol (MedChemExpress, HY-B0573) was administered by intraperitoneal injection to mice 0.5 h before, and 2.5 h and 5.5 h after 4-OHT-based Fos tagging in order to block β-adrenergic signalling throughout the tagging timeframe. In some experiments using DREADDs, mice were injected with CNO (1 mg kg−1) 10 min before the task in CtxA. For experiments in Fig. 2n in which CNO was used for silencing neuronal activity or propranolol was used for antagonizing β-adrenoreceptor signalling to test their roles in astrocyte Fos induction during fear memory recall, both were administered with consideration of their pharmacokinetics. Propranolol was given both before and after the behavioural session to ensure effective β-receptor blockade throughout the several-hour Fos-tagging window (Extended Data Fig. 3), given its short half-life78 (~1.5–2 h), whereas CNO was given once before the session owing to its prolonged effect79 (>6 h). Freezing was measured for 3 min in the CtxB chamber, which consisted of a rhombic partition (30 cm × 60 cm × 15 cm, 10 lux and 1% acetic acid odour) with a flat floor (Ohara).
Whole-brain imaging
Mice were deeply anaesthetized with isoflurane and transcardially perfused with PBS, followed by 4% PFA in PBS for fixation of brain tissues. The dissected brains were post-fixed overnight at 4 °C in 4% PFA in PBS and stored in PBS at 4 °C until embedding. To oxidize agarose, 2.25 g agarose (A6013, Sigma-Aldrich) was added to 100 ml of 10 mM sodium periodate (S1878, Sigma-Aldrich)/50 mM phosphate buffer and stirred gently for 2.5 h at room temperature in the dark. The oxidized agarose was filtered on a bottle-top vacuum filter (0.2 μm, 569-0020, Thermo Scientific) washed 3 times with phosphate buffer and resuspended in 50 ml of phosphate buffer to make 4.5% oxidized agarose solution. The oxidized agarose solution was boiled with a microwave oven to melt the agarose and kept at 60–65 °C. The brains were pat-dried and embedded in oxidized agarose solution with a plastic mould (18646A, Polysciences). For covalent crosslinking between oxidized agarose and the surface of the brain, borate/borohydride solution was prepared the day before use by adding 0.2 g sodium borohydride (452882, Sigma-Aldrich) in 100 ml of prewarmed (40 °C) borate buffer (50 mM borax, 221732, Sigma-Aldrich; 50 mM boric acid, B6768, Sigma-Aldrich; pH 9.0–9.5) in the chemical fume hood. The agarose block was removed from the mould, soaked in borate/borohydride solution to initiate crosslinking and incubated at 4 °C overnight. The block was washed and kept in phosphate buffer at 4 °C until imaging.
The agarose block was mounted on a glass slide using Loctite 401 glue immediately before imaging. STPT was performed with the TissueCyte 1400FC system (TissueVision) equipped with Coherent Chameleon Ultra II laser (Coherent) or Spectra-Physics Insight X3+ laser (MKS Spectra-Physics) and a Nikon 16× water-immersion objective (MRP07220, NA: 0.8). The laser wavelength was tuned at 1,000 nm. Brains were coronally sectioned with a built-in vibrating microtome at 50-μm thickness with a frequency of 60 Hz and a speed of 0.5 mm s−1. Four optical z-planes were taken at 50 μm, 62.5 μm, 75 μm and 87.5 μm below the cut surface of the tissue block. To scan an entire coronal plane, individual tile images were acquired (832 × 832 pixels per frame, resolution at 1.382 μm per pixel) by moving the stage between each tile. Following the full scanning of a coronal plane, a 50-μm section was cut away by vibrating microtome and imaging of a new cut surface repeated to acquire optical sections covering the entire brain from the caudal to the rostral side (~320 coronal sections). The raw image tiles were first trimmed and subjected to flat field correction, then stitched into 2D mosaic coronal section images using the Autostitcher software (TissueVision). The signals from the green channel (500–550 nm, mNG signal) and from the far-red channel (>600 nm, background) were simultaneously acquired, and the latter was used to subtract background from the green channel to enhance the signal to noise ratio. Each region of the whole brain was aligned and segmented according to the Mouse Brain Atlas (NeuroInfo, MBL Bioscience, BN-200). Owing to the high quality preservation of tissue architecture during imaging, we semi-automatically performed alignment to a standard mouse brain atlas, segmentation of 839 brain regions, and detection of single Fos-tagged mNG astrocytes (BAEs), defined by their territories that typically displayed diameters of approximately 40 to 60 μm with ~10-µm-wide cell bodies68 (Fig. 1a and Supplementary Videos 1 and 2). Consistent with our intersectional genetic approach, we rarely observed neuron-like mNG-positive cells, with larger cell bodies (>~20-μm diameter) and elongated processes (>~50-μm). We only found these cells in regions nearby ventricles and the midline where well-characterized GFAP-positive neural progenitor cells reside48,80, and they were excluded in subsequent analyses. As a result, using this brain-wide analysis approach we successfully quantified the density of BAEs in 677 brain regions that accounted for 80.7% of the whole brain.
Correlational analysis of Fos
+ cell counts
To observe correlation between Fos+ astrocytes and neurons, we used the same TRAP2 (Fos-iCreERT2) system28 used by Roy et al.5, but instead with a Cre-dependent mNG reporter expressed in Fos+ astrocytes, allowing comparison of Fos+ cell counts from anatomically matched regions between studies. The regions of interest were first narrowed to the 117 engram-signifiant regions defined by Roy et al. Then, to ensure analysis of bona fide astrocyte-tagged regions, areas with possible neuronal leakage (mNG+ neuron-like cells), particularly near ventricles were excluded (see above). In addition, high-level hierarchical structure areas (such as midbrain or thalamus) with extremely high total counts that skew correlation analysis were also excluded. In the end, this selection yielded 75 regions for subsequent correlational analyses. Changes in Fos+ astrocyte numbers (FC–NoFC and FR–NoFC) were calculated using the non-foot shocked control group as the baseline, consistent with Roy et al. Supplementary table 1 from Roy et al. provided region-by-region counts of Fos+ neurons in non-foot shocked controls, FC and FR groups, enabling us to extract the corresponding changes in Fos+ neuron numbers for each of the 75 regions. Pearson correlation analyses between these neuronal and astrocytic Fos+ cell count changes were performed.
Whole-brain visualization
For image visualization in 3D, low-resolution image stacks were created across all four channels. These stacks were downsampled to 25 μm per pixel resolution in the x and y axes, while the inter-slice distance was maintained at 50 μm, resulting in a voxel resolution of 25 × 25 × 50 μm3. To minimize high-frequency artifacts, median filtering and Gaussian smoothing were applied to the coronal images prior to downscaling. The processed 3D image stacks were stored in the NIfTI format, and the world coordinates were adjusted to align roughly with the Allen Mouse Brain Common Coordinate Framework v.3 (CCFv3)81. For registration to the CCFv3, the Allen STP-1675 template (filename: P56_Atlas.nii.gz; available at https://scalablebrainatlas.incf.org/mouse/ABA_v3#downloads) was used as the reference image. This template, generated from STPT averaged over 1,675 specimens, was combined with corresponding atlas annotations (filename: P56_Annotation.nii.gz). Image alignment was performed using the Advanced Normalization Tools (ANTs)82. An affine registration was first applied at 3 scales (downscaling factors of 4, 2 and 1) with Gaussian smoothing of 2, 1 and 1 voxels and 250, 200 and 50 iterations, respectively. This was followed by deformable (SyN) registration at four scales (downscaling factors of 8, 4, 2 and 1) with Gaussian smoothing of 4, 2, 1 and 1 voxels and 1,000, 500, 100 and 50 iterations, respectively, using mutual information as the metric. After computing the transformation, individual image stacks and atlas labels were mapped to and from the CCFv3 image space. After aligning with the NIfTI world coordinates, cell positions were mapped to the Allen template using the antsApplyTransformsToPoints function from ANTs. Brain regions from the Allen atlas annotations were then assigned to individual cells based on their locations in the CCFv3 image space.
Primary astrocyte culture
Astrocyte primary cultures were obtained as previously described with some modifications83. In brief, cerebral cortical tissues were isolated from P0–P1 neonatal mice and dissociated with a scalpel, gently agitated in 0.05% Trypsin-EDTA (Gibco, 25300-054), and rapidly strained with a Falcon 100 µm cell strainer (352360). Dissociated cells were seeded at a rate of four brains per bottle on poly-d-lysine-coated bottles. The cells were cultured in DMEM (Gibco, 11995073) supplemented with FBS (Cosmo Bio, CCP-FBS-BR-500) and penicillin-streptomycin. When the cells reached confluence after several weeks, the culture bottles were gently shaken at 120 rpm overnight at 37 °C to remove non-astrocytic cells. Astrocytes were then detached using Trypsin-EDTA, replated onto poly-d-lysine-coated bottles, and after 1–2 weeks, cultures were shaken again at 120 rpm for 4 h. Astrocytes were then re-seeded on coverslips in 12-well plates at a density of 1.0 × 105 cells per well, which were maintained in 5% CO2 at 37 °C, with the medium being changed every 2 to 3 days.
Primary astrocyte c-Fos analysis
Five days after re-seeding, primary astrocytes were incubated with tetrodotoxin (TTX at 1 µM, Fujifilm, 207-15901) to eliminate the potential effect of neuronal firing, for at least 5 min to allow adequate equilibration, together with the following inhibitors, blockers or chelators indicated for each experiment unless otherwise described: prazosin hydrochloride (MedChemExpress, HY-B0193A), atipamezole hydrochloride (Tokyo Chemical Industry, A2956), propranolol hydrochloride (MedChemExpress, HY-B0573), BAPTA-AM (SIGMA, A1076), and SQ 22536 (Tokyo chemical industry, Q0105). The cultures were then incubated with the following growth factors, neurotransmitters, neuromodulators or neuropeptides in 5% CO2 at 37 °C for 1.5 h: l-glutamic acid monosodium salt hydrate (Sigma-Aldrich, G5889), GABA (Sigma-Aldrich, A2129), ATP disodium salt hydrate from yeast (Nacalai tesque, 01072-24), l-noradrenaline hydrochloride (Sigma-Aldrich, 74480), dopamine hydrochloride (Sigma-Aldrich, H8502), acetylcholine chloride (Sigma-Aldrich, A6625), serotonin hydrochloride (Alfa Aesar, B21263.03), brain derived neurotrophic factor (BDNF, Fujifilm, 028-16451), neurotrophin-3 (Fujifilm, 146-09231, 50 ng ml−1), neurotrophin-4 (Fujifilm, 142-06634, 100 ng ml−1), forskolin (Fujifilm, 067-02191, 10 μM), nerve growth factor-β (NGFβ, Fujifilm, 141-07601, 100 ng ml−1), epidermal growth factor (EGF, Fujifilm, 059-07873, 100 ng ml−1), PDGF-AA (Fujifilm, 163–19731, 100 ng ml−1), PDGF-BB (Fujifilm, 164–24031, 100 ng ml−1), Ciliary Neurotrophic Factor (CNTF, Fujifilm, 032-23501, 100 ng ml−1), IGF1 (Fujifilm, 099-04511, 100 ng ml−1). The following agonist and ionophore were used: isoproterenol (MedChemExpress, HY-B0468) and ionomycin (LKT laboratories, I5752). Ninety minutes after incubation, primary astrocytes were fixed in 4% PFA (Nacalai Tesque) for 10 min, washed with PBS 3 times, permeabilized with 0.1% Triton X-100 (Nacalai Tesque) for 10 min and blocked with 1% NDS (Sigma-Aldrich, D9663) for 30 min. Primary astrocytes were then incubated overnight at 4 °C with the following primary antibodies: rabbit anti-c-Fos (1:1,000, Synaptic Systems, 226003), rat anti-GFAP (1:1,000, Thermo Fisher Scientific, 13-0300), rabbit anti-IBA1 (1:1,000, Fujifilm, 019-19741), chicken anti-TUJ1 (1:1,000 Novus Biologicals, NB100-1612), mouse anti-OLIG2 (1:100, Millipore, MABN50). The cultures were washed 3 times in PBS for 10 min each, then incubated with DAPI (1:10,000, Sigma-Aldrich, D9542) and the following secondary antibodies (1:1,000) in PBS containing 1% NDS for 1 h at room temperature: Alexa Fluor 488 donkey anti-rat (abcam, ab150153), Alexa Fluor 568 donkey anti-mouse (abcam, ab175700), Alexa Fluor 568 donkey anti-rabbit (Abcam, ab175692), Alexa Fluor 647 donkey anti-rabbit (abcam, ab150067), Alexa Fluor 647 donkey anti-chicken (Jackson ImmunoResearch, 703-605-155). Fluorescent images were taken using a 20× objective lens (UPlanXApo 20×, Olympus) with a confocal laser-scanning microscope (Olympus, FV3000 with Fluoview). Immunoreactivity quantification of c-Fos was performed using ImageJ. The c-Fos brightness of GFAP-positive astrocytes were measured by the intensity of ROIs that encompassed the cell nuclei. In some experiments involving a heterologous expression of mGluR3, we applied AAV2/5-GfaABC1D-Grm3 (800,000 multiplicity of infection) 5 h after re-seeding for expressing mGluR3 in primary astrocytes and incubated for 5 days before pharmacological experiments. Ninety minutes after stimulation with 0.1 µM NA and/or 100 µM glutamate in the presence of 1 µM TTX, primary astrocytes were fixed and stained as described above using primary antibodies: rabbit anti-mGluR3 (1:500, abcam, ab166608) and mouse anti-c-Fos (1:50, Santa Cruz Biotechnology, sc166940). It is noteworthy that the mouse anti-c-Fos antibody, different from the rabbit anti-c-Fos antibody used in other experiments, exhibited overall low immunoreactivity. However, this did not affect the quantification of c-Fos mobilization. Fluorescent imaging and quantification of c-Fos intensity were performed as described above.
Fibre photometry
AAVs for expressing the noradrenaline biosensor GRABNE2h on the neuronal cell surface, or calcium indicator RCaMP3 in astrocytes with cAMP sensor cAMPinG1 in astrocytes were unilaterally injected into the basolateral amygdala. Optical fibres were implanted above the injection site two weeks post-injection to capture emission of fluorescence signals, with recordings conducted one week after fibre implantation. For fluorescence signal collection, mice were tethered to a patch cable, and a 465 nm LED light (Doric, 40–50 µW at the tip of patch cable) or a 560 nm LED light (Doric, 40–50 µW at the tip of patch cable) was used to excite the signal, with an isosbestic 405 nm LED light used to correct for movement artifacts. Mice tethered to the patch cable were habituated in a testing chamber for 5 min on the day of recording. A time-division multiplexing scheme was used, where the 465 nm, 560 nm and 405 nm LED lights were emitted alternately at 20 Hz (turned on for 24 ms and off for 26 ms), with the timing precisely controlled by a programmable pulse generator (Master-9, AMPI). Each excitation light was reflected by a dichroic mirror and coupled into an optical fibre cable (Doric, 200 μm in diameter, 2 m in length, NA 0.57) through a pinhole (200 μm in diameter). The fluorescence signals were detected by a photomultiplier tube with a GaAsP photocathode (H16722-40; Hamamatsu Photonics) and digitized by a data acquisition module (USB-6341, National Instruments). A custom-written LabVIEW program extracted signals at a sampling frequency of 1 kHz in real time. Fluorescence signals collected during each light pulse were averaged, after dropping the first and last 2 samples (1 ms each) to eliminate potential noise caused during the alternation of LED lights. The data were smoothed using moving average and corrected for bleaching correction by fitting to least-squares linear. The fluorescence response was calculated (fitted 465 + 1)/(fitted 405 + 1) − baseline. The baselines of GRABNE2h and astrocyte cAMPinG1 signals were identified during periods of no spontaneous signals in home cages for 1–2 min before the context exposure. Signal analyses were performed in Python (v.3.0.0) using JupyterLab (v.3.6.7, Project Jupyter). Numerical computations and data handling were conducted using NumPy84 (v.1.26.4) and Pandas (v.2.1.4, NumFOCUS). The area under the curve of fluorescence traces was calculated with scikit-learn (sklearn, v.1.2.2, RRID:SCR_002577), and half-decay times of signal responses were extracted. Astrocytic RCaMP3 signals were bandpass filtered between 0.0003 and 1 Hz with SciPy85 (v.1.11.4). Peaks of Ca2+ signals were defined as data points exceeding 4× s.d. above the baseline mean, with baseline noise estimated from ≥20 s intervals lacking spontaneous activity in home cage recordings before context exposure. Signal traces were visualized with Matplotlib86 (v.3.8.0).
Acute brain slice preparation for astrocyte intracellular Ca2+ imaging
Coronal amygdalar slices were prepared from adult mice with AAV injection26,87. In brief, animals were deeply anaesthetized with isoflurane and decapitated with sharp shears. The brains were placed and sliced in ice-cold modified artificial cerebrospinal fluid (ACSF) containing the following (in mM): 194 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4 and 10 d-glucose, saturated with 95% O2 and 5% CO2. A vibratome (DSK NLS-MT) was used to cut 300-µm brain sections. The slices were allowed to equilibrate for 30 min at 32–34 °C in normal ACSF containing (in mM); 124 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4 and 10 d-glucose continuously bubbled with 95% O2 and 5% CO2. Slices were then stored at 21–23 °C in the same buffer until use. All slices were used within 2–6 h of slicing. Cells for all the experiments were imaged with a 16× water-immersion objective lens (Nikon, N16XLWD-PF, MRP07220, NA: 0.8) with Nikon two-photon laser-scanning microscope (A1R MP) equipped with a 920 nm wavelength laser (Alcor, Spark Lasers). To image GCaMP6f signals, astrocytes located in the LA/B and typically ∼20 to ∼30 µm below the slice surface were selected for imaging. Images were acquired at 1 frame per second. Slices were maintained in ACSF (124 mM NaCl, 4.5 mM KCl, 1 mM MgCl2, 1.2 mM NaH2PO4, 26 mM NaHCO3, 10 mM d-glucose and 2.0 mM CaCl2) through a perfusion system. Phenylephrine (PE, Tocris Bioscience 2838) was applied in the bath in the presence of 300 nM tetrodotoxin (Cayman Chemical 14964) were applied in the bath at least 5 min prior to recording to allow adequate equilibration. A constant flow of fresh buffer perfused the imaging chamber at all times. Spontaneous Ca2+ activity was recorded for 3 min (0 µM PE), followed by a 2-minute bath application of 1 µM PE, a 5-min washout, a 2-min application of 3 µM PE, and a 5-min washout. Ca2+ signals were processed in ImageJ (NIH) and presented as the relative change in fluorescence (ΔF/F).
scRNA-seq and analysis
Mice were anaesthetized with isoflurane 90 min after the initiation of behavioural paradigms and transcardially perfused with PBS (30 ml for 2 min). The amygdala was freshly dissected out using a Brain Matrix (Ted Pella, 15067) and was placed into cold Hanks’ balanced salt solution (HBSS) (14025092, Gibco) containing 10 mM HEPES (17514-15, Naclai Tesque), 0.54% glucose (16806-25, Naclai Tesque), 5.0 µg ml−1 actinomycin D (A1410, Sigma-Aldrich), 10 µM triptolide (T3652, Sigma-Aldrich) and 27.1 µg ml−1 anisomycin (A9789, Sigma-Aldrich), as previously described88,89. Then, the tissue block was incubated for 15 min at 37 °C in 3 ml enzyme solution (HBSS, 10 mM HEPES, 0.54% glucose, 0.5 mM EDTA (15575020, Invitrogen), 1.0 mM l-cysteine (10309-41, Naclai Tesque), 5.0 µg ml−1 actinomycin D, 10 µM triptolide and 27.1 µg ml−1 anisomycin) containing 20 U papain per mouse (LS003127, Worthington Biochemical). The tissue was then homogenized by passing through a 18 G needle and was further incubated for 15 min at 37 °C. Following homogenization using a 20 G needle, the resulting cell suspension was centrifuged at 500g for 5 min at 4 °C. The homogenate was separated by gradient centrifugation with 30% Percoll (P1644, Sigma-Aldrich) in PBS at 500g for 25 min at 4 °C (no brake). The pellet containing astrocytes at the bottom of the tube was then collected and washed once with PBS containing 2% FBS and 10 mM EDTA before staining. Fc receptors were blocked with Fc block (2.4G2, BD Bioscience) for 10 min at 4 °C before incubation with primary antibodies. Cells were stained with antibodies directed against CD11b-BV786 (1:200, M1/70, BD Bioscience), CD45-APC-Cy7 (1:200, 30-F11, BioLegend), O1-eFluor660 (1:200, 50-6506-82, eBioscience) and ASCA2-PE-Cy7 (1:200, 130-116-246, Miltenyi Biotec) for 40 min at 4 °C. Additionally, cells were treated with hashtag antibodies to label source samples (TotalSeqB0301-B0310, B0312 and B0314, BioLegend). After washing, cells were sorted using a CytoFLEX SRT (Beckman Coulter). Data were acquired with CytExpert software (Beckman Coulter). Post-acquisition analysis was performed using FlowJo software, version 10.9.0. Single-cell libraries were generated and sequenced on the Illumina NovaSeq 6000 sequencer. Sequence reads were processed and aligned to the mouse genome (mm10, GENCODE vM23/Ensembl98; https://cf.10xgenomics.com/supp/cell-exp/refdata-gex-mm10-2020-A.tar.gz) using CellRanger 3.0. Initially, genes expressed in <3 cells and cells with <100 genes and mitochondrial reads <6% were excluded from the analysis. Pericytes were removed from amygdalar cells based on Pdgfrb. Hashtag oligos (HTOs) were demultiplexed using HTODemux and singlet cells were used for subsequent analysis. Principal component analysis was performed on the expression data matrix using the Seurat. Thirty principal components were used for generating tSNE plots. Transcriptomic clusters were identified from a total 1,903 amygdalar astrocytes with resolution set to 0.4. Differentially expressed gene candidates from a comparison of Adrb1-positive and Adrb1-negative astrocytes were estimated using Mann–Whitney–Wilcoxon test.
RNAscope
Cryosections were prepared as described above and stored at −80 °C. RNAscope in situ hybridization was performed using RNAscope Multiplex Fluorescent Reagent Kit v.2 with TSA Vivid Dyes (ACDBio, 323270), according to the manufacturer’s instructions. The sections were washed for 5 min with PBS, and then heated at 60 °C for 30 min before post-fix. After dehydration in an ethanol series, hydrogen peroxide was applied for 10 min at room temperature. The sections were washed with ddH2O twice, and then incubated in Target Retrieval Reagents for 5 min at 90–100 °C. A wash with ddH2O for 15 s was followed by a dehydration with 100% ethanol for 3 min and dried at room temperature. Then, the sections were incubated with Protease III for 30 min at 40 °C. The sections were washed with ddH2O twice and then incubated with probes Mm-Fos (ACDBio, 316921), Mm-Adra1a (ACDBio, 408611), Mm-Adrb1-C2 (ACDBio, 449761-C2), Mm-Igfbp2 (ACDBio, 405951) and Mm-Slc1a3-C3 (ACDBio, 430781-C3) for 2 h at 40 °C. Thereafter, the sections were washed twice with wash buffer for 2 min between incubations at 40 °C. The sections were incubated in AMP1 for 30 min, AMP2 for 30 min and AMP3 for 15 min at 40 °C, then incubated in horseradish peroxidase (HRP) for 15 min, fluorophore for 30 min and HRP-blocker for 15 min at 40 °C for each channel. In the staining of mCherry, slices were washed in 0.1 M PBS 3 times for 10 min each, followed by immunohistochemistry that was performed as described above. Following primary and secondary antibodies used: rabbit anti-RFP (1:250, Rockland, 600-401-379), Alexa Fluor 568 donkey anti-rabbit (1:1,000, Abcam, ab175692). Finally, the sections were incubated with DAPI at room temperature, removed DAPI, and mounted in Prolong Gold Antifade Reagent (Invitrogen, P36934). Images were obtained in the same way as immunohistochemistry described above except with a step size of 0.5 µm. Images were processed with ImageJ. Astrocyte somata were delineated using Slc1a3 signals. The intensity of Fos, Adra1a, Adrb1 and Igfbp2 signals within the somata were measured. To account for differences in somata sizes, the signals were normalized using Slc1a3. Subsequently, the normalized signals were z-scored within each sample to evaluate gene expression across a heterogeneous cell population. This approach facilitated the robust identification of cells exhibiting high gene expression relative to the population mean. In Fig. 4e,f, adrenoreceptor-positive cells were identified based on scRNA-seq data, selecting the top 50% of cells ranked by Adra1a expression and the top 20% of cells ranked by Adrb1 expression. Co-expression index of Adra1a and Adrb1, calculated by relative expression level of Adra1a to NoFC median multiplied by relative expression level of Adrb1 for each cell. In Fig. 4j, the correlation analysis of Fos and Adrb1, expression levels were evaluated using z-scores of RNAscope signal intensity to assess gene expression across a heterogeneous population of cells, enabling robust identification of cells with high gene expression relative to the population mean. Specifically, we identified astrocytes with z-scores greater than 2 in RNAscope Fos signal intensity as Fos-high astrocytes, capturing 3% of the astrocyte population with significantly elevated Fos expression, consistent with scRNA-seq data in Fig. 4c where Fos-positive astrocytes occupied 4% of the total astrocytes analysed. Similarly, Adrb1-high astrocytes were identified using z-scores greater than 0.5, capturing 26% of astrocytes, also in line with scRNA-seq data in Fig. 4c where Adrb1-positive astrocytes occupied 20% of the total astrocytes analysed. In addition, Igfbp2-high astrocytes were identified using z-scores greater than 0.5, capturing 24% of all astrocytes in Extended Data Fig. 12d,e, which is consistent with scRNA-seq data indicating that Igfbp2-positive astrocytes occupy 24% of the total astrocytes analysed.
Statistical analysis
Most statistical tests were run in Prism 10.1.2 (GraphPad). The specific tests used are indicated in the figure legends and Supplementary Table 1. All tests were two-sided unless otherwise noted. Astrocyte Fos count analyses across groups for each brain region were run with R v.4.3.2 on RStudio v.2023.12.1.402 and relied on the tidyverse, car, dunn.test and dplyr packages and base R functions aov(), TukeyHSD() and p.adjust(). The data were first analysed with Levene’s test for homogeneity of variance. Brain regions that passed Levene’s test (P > 0.05) were followed by a one-way ANOVA test with Tukey’s honest significant difference test and regions that failed Levene’s test (P < 0.05; no homogeneity of variances) were followed by the Kruskal–Wallis test with Dunn’s post hoc test and Bonferroni adjustment. False discovery rates were calculated across groups and brain regions from post hoc P values using the Benjamini–Hochberg method.
Mice were randomly assigned to experimental groups, and mice assigned to different experimental conditions were run in parallel. Mouse experiments and analyses were done blinded to group allocation. Tissue samples that yielded insufficient cell counts (less than 30 cells) during scRNA-seq quality control were excluded from the study. The results of statistical comparisons, n numbers and P values are shown in the figure panels or figure legends along with averages. n is defined as the numbers of cells, sections or mice throughout on a case-by-case basis; the unit of analysis is stated in the text or in each figure legend. In the figures, summarized data are shown as mean ± s.e.m. with a scatter plot and P values are indicated by asterisks: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.