Materials
All chemicals were from Sigma Aldrich unless indicated differently. Potassium superoxide (KO2), potassium perchlorate (KClO4, ≥99.99%), lithium bis(trifluoromethanesulfonyl)imide (LiTFSI, 99.99%) and tetrabutylammonium bis(trifluoromethanesulfonyl)imide (TBATFSI, ≥99.0%) were dried under reduced pressure for 24 h at 100 °C. Decamethylferrocene (DMFc), tris[4-(diethylamino)phenyl]amine (TDPA), N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD), ferrocene (Fc), ferrocenium tetrafluoroborate (FcBF4), 9,10-diphenylanthracene (DPA), 1,4-bis(diphenylamino)benzene (DPAB), thianthrene (ThA), 1,4-dimethoxyanthracene (DMeOA, Acros Organics), 1,4-di-tert-butyl-2,5-dimethoxybenzene (tMeOB, BLDpharm) and 5,10-dihydro-5,10-dimethylphenazine (DMPZ, TCI chemicals) were used as received. N-methyl phenothiazine (MPT), 1,4-diazabicyclo[2.2.2]octane (DABCO) were sublimated. 18-Crown-6 and tetrafluorobenzoquinone (F4BQ) were recrystallized from ethanol. Tetraethylene glycol dimethyl ether (TEGDME, ≥99%) was dried over lithium, distilled under vacuum and further stored over activated 3 Å molecular sieves. Acetonitrile (MeCN, 99.8% anhydrous) was stored over activated molecular sieves. Both solvents had a water content below 5 ppm as determined by Karl–Fischer titration (Mettler Toledo). All non-aqueous experiments were performed in an Ar-filled glovebox (Vigor) or hermetically sealed setups without air exposure. The structures of the mediators and their redox potentials are shown in Extended Data Fig. 2.
Electrochemistry and mediator oxidation
Electrochemistry experiments were performed using a BioLogic potentiostat (SP-300 and MPG-2). Cyclic voltammetry was performed with a glassy carbon disk as the working electrode and a glassy carbon rod as the counter electrode in a one-compartment glass cell. Partially delithiated Li(1–x)FePO4 (LFP, MTI), separated by a Vycor glass frit, was used as the reference electrode. DMFc/DMFc+ was used as the internal standard and converted using \({E}_{{\rm{DMFc}}/{{\rm{DMFc}}}^{+}}^{\circ }={E}_{{\rm{Li}}/{{\rm{Li}}}^{+}}^{\circ }+3.16\,{\rm{V}}={E}_{{\rm{K}}/{{\rm{K}}}^{+}}^{\circ }\)\(+3.02\,{\rm{V}}.\) DMeOA, tMeOB, DPA and ThA were electrochemically oxidized in an H-cell. The oxidation compartment contained a Pt working electrode, the reference electrode and 5 ml MeCN containing 2 mM RM and 10 mM KClO4. The reduction compartment contained Ni foam as a counterelectrode with 5 ml of 100 mM 1,4-benzoquinone (sublimed) in MeCN. A K+ selective ion-exchange membrane separated the two compartments. RMs were oxidized galvanostatically to 80% of their total capacity. DMFc, TDPA, TMPD, DMPZ, MPT and DPAB were oxidized using 1 equiv. NOBF4 in MeCN. After 3 h stirring, they were precipitated with cold diethyl ether, filtered and dried under a vacuum at 30 °C for 12 h. F4BQ is the oxidized form itself.
Measurements of kinetics
Kinetics of mediated superoxide oxidation were measured using UV–Vis spectroscopy using an Avantes AvaSpec-HSC spectrometer with AVALIGHT-DH-S-BAL light source and fibre optics to perform measurements inside the glove box. Pure KO2 powder was pressed into about 0.5 mm thick pellets using a 7 mm die set and a hand press (PIKE). In a 10-mm quartz cuvette (Hellma), a KO2 pellet was placed in a polytetrafluoroethylene frame for alignment, followed by a magnetic stirring bar and then the cuvette was sealed with a gas-tight injection lid. RMox solution containing 10 mM KClO4 was then injected using a gas-tight syringe (Hamilton). Consumption of RMox was followed except for F4BQ, in which formation of RMred was followed (Extended Data Fig. 7). Data and error bars are presented as mean ± s.d. (n ≥ 3). Error bars appear asymmetric on an ln(k) scale. Repetitions mean that each time a new portion of RMox was produced by electrochemically oxidizing a portion of RMred or by dissolving the chemically produced oxidized form. Differences in the magnitude of the error bars among mediators arise from their large chemical diversity and specific reactivities. For example, Fc+ and reduced quinones react with O2, or high-voltage RMox shows limited long-term stability in the electrolyte. For the latter, it was checked that degradation was at least several times slower than the oxidation of KO2.
Kinetics of Li+-induced superoxide disproportionation in TEGDME were measured by placing KO2 powder in a closed reaction vessel equipped with a pressure sensor (Omega, PAA35X) and injecting the Li+ electrolyte using a syringe through a septum (Extended Data Fig. 8).
3O2 yields and 1,270 nm emission measurements
3O2 yields on mediated superoxide oxidation were measured using mass spectrometry, as detailed previously30. The RMox solutions were injected using a gas-tight syringe, and the measurement continued until the O2 signal ceased. We used the 1O2-specific NIR emission at 1,270 nm from the decay of 1O2 to 3O2 to determine 1O2 yields and lifetimes as detailed previously30. The signal was recorded from the detector using an oscilloscope (Pico Technology) and at a gain of 820 V (control voltage). Extended Data Fig. 6 shows examples of the recorded signal during mediated oxidation, as well as Li+– and H+-induced disproportionation.
From oxidation rates to 3O2 yields and NIR emission intensities
We use the yields of 3O2/RMox and the normalized NIR intensities in Fig. 2b,c to prove that the two kinetic parabolas correspond to 3O2 and 1O2 evolution. To rationalize their assignment, we use a minimal model to calculate expected 3O2/RMox and NIR emission intensities based on formation rates and 1O2 decay processes. We considered the following processes: (1) 3O2 and 1O2 formation rates (k3 and k1) as given by the two kinetic parabolas in Fig. 2a; (2) physical and reactive 1O2 quenching by the solvent; (3) physical and reactive 1O2 quenching by the reduced mediators RMred. Note that the same processes with RMox are typically negligible in comparison because of the electron demand of these processes36; and (4) 3O2 losses (3O2/RMox < 1) resulting from reactive quenching of 1O2 with solvent or mediator.
Losses in 3O2/RMox in Fig. 2b result from incomplete physical quenching of 1O2 to 3O2 due to reactions of 1O2 with electrolyte or RM. Hence, we start by considering the decay routes. There are multiple decay routes, as shown in Extended Data Fig. 3f, and only a small fraction of the total 1O2 can be detected by the NIR emission at 1,270 nm (refs. 12,37). First, interactions of 1O2 with solvent will result in electronic-to-vibrational (e–v) deactivation and reactions with the solvent. The first-order 1O2 decay rate constant kD = kd,r + kd is composed of a reactive fraction kd,r and a non-reactive fraction kd. Second, electron-rich species, such as reduced mediators, exert charge transfer quenching; however, they may also react with the electrophilic 1O2 along pathways depending on the particular chemistry36. The rate constant kQ = kq,r + kq is equally composed of a reactive fraction kq,r and a non-reactive fraction kq. Third, radiative decay of 1O2 to 3O2 with the emission of a 1,270 nm photon.
We measured the rate constants kD and kQ using the luminescence lifetime. An O2-saturated solution containing Rose Bengal (absorbance of about 0.1 in a 1 cm fluorescence cuvette) was illuminated using a pulsed Coherent OBIS 561 nm laser. The lifetime (τ) and the decay constant (k = 1/τ) of 1O2 were measured using the NIR detector. τ was obtained by fitting the decay profile with exp(–t/τ) (Extended Data Fig. 3a). τ in the absence of a quencher relates to the solvent quenching rate constant kD. kQ is obtained by plotting 1/τ against the RMox concentration \({c}_{{{\rm{RM}}}^{{\rm{ox}}}}\) (Extended Data Fig. 3b,c). Similar to previously reported quenchers12, kQ decreases with \({E}_{{\text{RM}}^{\text{ox}/\text{red}}}^{\circ }\) following a trendline with a slope of about –103.5 V−1. The logarithmic dependence of the non-radiative fraction kq is explicable by the required partial charge transfer from the e–-rich quencher to the 1O2, which makes quenchers with lower redox potentials more efficient12. We consider that the reactive fraction kq,r depends similarly on redox potential, given that the addition reactions equally require charge transfer from the substrate to 1O2 (refs. 12,36). The scattering of kQ around the trendline arises from the large chemical diversity of the used mediators. We used both the trendline and the individual values of kQ for the prediction of NIR intensities in Fig. 2c. As for the fraction of reactive deactivation, kd,r/kD and kq,r/kQ, these can be determined from 3O2 consumption12, which we do below when simulating values of 3O2/RMox.
The surface area A of the KO2 powder, needed for the calculations, was determined by analysing optical images (Extended Data Fig. 3d). KO2 powder was dispersed in TEGDME, sonicated, a drop was placed between a microscope slide and a cover slip and sealed air-tight. Images were acquired with transmitted light on a Nikon Ti2E-01 inverted microscope using a Plan Apo λ 40×/0.95 DIC (Differential Interference Contrast) air PFS (Perfect Focus System) objective lens, resulting in a pixel size of 0.183 μm. The images were analysed using the ilastik pixel classification and object classification workflows (https://www.ilastik.org/), resulting in a histogram of particle sizes (Extended Data Fig. 3e). The surface area was calculated by assuming spherical particles, yielding A = 0.23 ± 0.04 m2 g–1.
As quantification of absolute 1O2 yields from NIR emission is not straightforward, we normalized the values. The NIR intensity at time t after bringing KO2 in contact with RMox will be proportional to the 1O2 concentration, \({I}_{1,270{\rm{nm}}}(t)\propto {c}_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2}}(t)\). The 1O2 formation rate by mediated KO2 oxidation is \({\nu }_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2}}(t)=A\cdot {k}_{1}\cdot {c}_{{{\rm{RM}}}^{{\rm{ox}}}}(t)\), with k1 being the rate constant as given in Fig. 2a. A is constant because the experiments were performed with a large excess of KO2 over RMox. \({c}_{{{\rm{RM}}}^{{\rm{ox}}}}\) and \({c}_{{{\rm{RM}}}^{{\rm{red}}}}\) are given by \({c}_{{{\rm{RM}}}^{{\rm{ox}}}}(t)={c}_{{{\rm{RM}}}^{{\rm{ox}}}}(0)\cdot {{\rm{e}}}^{-{k}_{{\rm{tot}}}\cdot A\cdot t}\) and \({c}_{{{\rm{RM}}}^{{\rm{red}}}}(t)={c}_{{{\rm{RM}}}^{{\rm{ox}}}}(0)-{c}_{{{\rm{RM}}}^{{\rm{ox}}}}(t)+\)\({c}_{{{\rm{RM}}}^{{\rm{red}}}}(0)-{\nu }_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2},{\rm{q}},{\rm{r}}}(t)\cdot t\), where ktot = k1 + k3. The term \({\nu }_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2},{\rm{q}},{\rm{r}}}(t)\) is the rate at which 1O2 reacts with RMred as detailed below. 1O2 formation and decay will balance37, which results in the presence of solvent and mediator quenching in \({c}_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2}}(t)={\nu }_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2}}(t)/({k}_{{\rm{d}}}+{k}_{{\rm{Q}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{red}}}}(t))\). kQ is either the measured value for the particular mediator or the trendline in Extended Data Fig. 3c. Finally, analogous to the experiment where we integrate the NIR signal until it ceases, we arrive at the expected NIR emission:
$${I}_{1270{\rm{nm}},{\rm{cum}}}\propto {\int }_{0}^{\infty }\,\frac{A\cdot {k}_{1}\cdot {c}_{{{\rm{RM}}}^{{\rm{ox}}}}(t)}{{k}_{{\rm{D}}}+{k}_{{\rm{Q}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{red}}}}(t)}{\rm{d}}t.$$
(3)
To simulate losses of 3O2 (3O2/RMox < 1 in Fig. 2b), we used the rate of reactive 1O2 decay \({\nu }_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2},{\rm{r}}}(t)=({k}_{{\rm{d}},{\rm{r}}}+{k}_{{\rm{q}},{\rm{r}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{red}}}}(t))\cdot {c}_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2}}(t)\) to define fr as the reactive fraction of the total 1O2 decay rate
$${f}_{{\rm{r}}}=\frac{{k}_{{\rm{d}},{\rm{r}}}+{k}_{{\rm{q}},{\rm{r}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{red}}}}(t)}{{k}_{{\rm{D}}}+{k}_{{\rm{Q}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{red}}}}(t)}.$$
(4)
\({\nu }_{{}^{1}{\rm{O}}_{2},{\rm{r}}}(t)\) equals the total mediated 1O2 formation rate times fr: \({\nu }_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2},{\rm{r}}}(t)={f}_{{\rm{r}}}\cdot A\cdot {k}_{1}\cdot {c}_{{{\rm{RM}}}^{{\rm{ox}}}}(t)\). As some of the RMred reacts, its loss is accounted for using \({\nu }_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2},{\rm{q}},{\rm{r}}}(t)={k}_{{\rm{q}},{\rm{r}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{red}}}}(t)\cdot {c}_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2}}(t)={f}_{{\rm{q}},{\rm{r}}}\,\cdot A\cdot {k}_{1}\cdot {c}_{{{\rm{RM}}}^{{\rm{ox}}}}(t)\), where \({f}_{{\rm{q}},{\rm{r}}}=\,{k}_{{\rm{q}},{\rm{r}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{red}}}}(t)/({k}_{{\rm{D}}}+{k}_{{\rm{Q}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{red}}}}(t))\). Calculations of fr, fq,r and \({c}_{{{\rm{RM}}}^{{\rm{red}}}}(t)\) were iterated until convergence was achieved. Finally, 3O2 yields are
$${{}^{3}{\rm{O}}}_{2}/{{\rm{RM}}}^{{\rm{ox}}}=1-{\int }_{0}^{\infty }{f}_{{\rm{r}}}\cdot A\cdot {k}_{1}\cdot {c}_{{{\rm{RM}}}^{{\rm{ox}}}}(t){\rm{d}}t/{\int }_{0}^{\infty }A\cdot {k}_{{\rm{tot}}}\cdot {c}_{{{\rm{RM}}}^{{\rm{ox}}}}(t){\rm{d}}t.$$
(5)
The fractions of reactive deactivation, kd,r/kD and kq,r/kQ were obtained by fitting the measured values of 3O2/RMox in Fig. 2b with the simulated ones from equation (5). The simulated 3O2/RMox are plotted as the dashed line in Fig. 2b.
Deviations between simulated and measured values may result from the simplicity of the model. The main simplification is as follows: (1) kQ was measured in homogeneous solutions of RMred and the model calculates bulk concentrations \({c}_{{{\rm{RM}}}^{{\rm{ox}}}}\) and \({c}_{{{\rm{RM}}}^{{\rm{red}}}}\), but during the heterogenous KO2 oxidation, bulk and surface concentrations of RMox and RMred differ. (2) We fitted a common fraction of mediator reactivity (kq,r/kQ) for all mediators, causing kq,r and kQ to decrease exponentially with growing \({E}_{{\text{RM}}^{\text{ox}/\text{red}}}^{\circ }\). As a trend, this is justified given that both reaction and charge transferquenching require e– transfer, but individual reactivity will vary because of the large chemical diversity of mediators36. (3) We did not account for quenching by O2 and \({{\rm{O}}}_{2}^{-}\). (4) Some RMs show further reactivities, which can cause 3O2 loss: Fc+ reacts with O2 (ref. 38), TDPA gets in contact with O2 spontaneously oxidized to TDPA+ and TDPA2+ (ref. 18), and reduced quinones bind to O2 (ref. 39).
Proton-induced disproportionation
Six types of buffers were used for proton-induced superoxide disproportionation. Britton–Robinson (BR) buffers were prepared with 0.1 M acetic acid, 0.1 M boric acid and 0.1 M phosphoric acid. NaOH (1 M) and HCl (1 M) were used to adjust the pH. Phosphate-buffered saline (PBS) solution and aqueous PBS powder solution (for pH 7.4) were added and the pH was adjusted using 1 M NaOH or 1 M HCl. Citrate buffers were prepared using citric acid and trisodium citrate dihydrate. To adjust the pH, we varied the concentrations of citric acid and trisodium citrate dihydrate. Acidic buffers of KCl and HCl (pH 1 and 2) were prepared by mixing 0.2 M KCl with 0.2 M HCl and adjusting the volume. Neutral to basic buffers of KH2PO4/NaOH (pH 7.1 and 10.8) were prepared by mixing 0.1 M KH2PO4 with 0.1 M NaOH and adjusting the volume accordingly. To trap the hydrogen peroxide (H2O2) produced, to eliminate the possibility of forming 1O2 by peroxoacids40, titanium(IV)oxysulfate solution (TiOSO4, 15 wt% in dilute sulfuric acid), 1 M NaOH was used to adjust the pH of TiOSO4 solutions to pH 1.2 and 1.55.
Figure 4c shows 1O2 yields still increasing when the pH is below 4.8, the pKa of O2−. KO2 hydrolysis increases the pH in aqueous media according to KO2 + H2O → HO2 + K+ + OH–. Buffer capacities were selected so that the amount of KO2 did not significantly affect the resulting pH after the reaction. However, despite the buffers, the local pH at the reaction site will be higher than the average. The importance of buffering for a local pH close to the average is evident in control experiments without a buffer. Even at a pH of 1.5, we could not detect 1O2 in an unbuffered H2SO4 solution, whereas we could in the buffered one (Extended Data Fig. 6e). Therefore, the 1O2 yields in Fig. 4c result from a higher local pH.
Driving forces on superoxide oxidation
Superoxide experiences a broad range of oxidizing conditions to liberate oxygen, but explanations for why and to what extent certain oxidizing redox couples evolve 1O2 have been unknown. Extended Data Fig. 4 shows the driving forces for superoxide oxidation with various redox couples. The driving forces are shown in comparison with the Marcus kinetic parabola in ether and acetonitrile solvent from Figs. 1 and 2. Li+– and H+-induced disproportionation are shown in Figs. 3 and 4. The other examples we discuss in Extended Data Fig. 4 arise from superoxide in contact with CO2 or organic peroxides, with relevance for energy storage and biology1,30,41.
Considering CO2 first, we have previously shown that CO2 in contact with O2− yields 1O2, but the energetics were unknown30. CO2 in contact with O2− is known to form peroxomonocarbonates and peroxodicarbonates by repeated uptake of CO2 by O2−. Intermediate peroxocarbonate species may be reduced by O2−, which releases O2 (refs. 41,42,43,44). However, the O2 spin state has previously not been considered. Extended Data Fig. 4c shows likely redox couples of oxidized/reduced peroxocarbonate species, but their redox potentials are not established experimentally. A previous study has shown, using density functional theory (DFT), that depending on the cation present and the solvent, the particular peroxocarbonate redox couples that oxidize O2− to O2 differ4, but likely involve CO4•–/CO42–, LiCO4•/LiCO4–, Li2C2O6/Li2C2O6– or Li3C2O6/Li3C2O6–. The calculations have shown reaction energies relative to the O2−/3O2 between about 1 and 1.4 eV. These driving forces, hence, explain 1O2 formation from CO2 in contact with O2− (Extended Data Fig. 4b).
1O2 formation has been examined for exposure of superoxide to organic peroxides, given their occurrence in biological systems45. No reaction was observed with alkyl peroxides, whereas acyl peroxides yielded 1O2 (refs. 45,46); however, this has not been connected with driving forces for superoxide oxidation. Nucleophilic attack of O2− on acyl peroxides forms an acyl radical and carboxylate (Extended Data Fig. 4d). The potential for the acyl radical/carboxylate redox couple has been reported between 1.5 V and 1.7 V compared with SHE (ref. 23) (1.95–2.05 V compared with O2/O2−), which explains 1O2 formation according to Extended Data Fig. 4a. Alkyl peroxides (ROOR) such as di-tert-butyl, dicumyl and di-n-butyl peroxide have been reported not to form 1O2 (ref. 45). This can be understood by ROOR only breaking down to RO– and RO• at low potentials (–1.31 V to –1.15 V compared with SHE, that is, –0.86 V to –0.70 V compared with O2/O2−) (ref. 47). The RO•/RO– couple (–0.06 V to 0.04 V compared with SHE, that is, 0.39–0.49 V compared with O2/O2−) could, if formed, oxidize O2−, but not to 1O2.
Proton-induced disproportionation that yields low but non-negligible 1O2 yields at a high pH of about 11 is consistent with reliable theoretical and experimental works. Using a high-level ab initio method, a previous study17 found that the reaction HO2 + O2− → O2 + HO2 can proceed by both the singlet and triplet pathways, with the singlet pathway being only about 0.3 eV endoergic. Equivalent experiments exposing KO2 to H2O2 in toluene found about 0.2% 1O2 production, as measured using a chemical trap14. In this experiment, H2O2 was the proton source to first form HO2 (H2O2 + O2− → HO2− + HO2). The conditions in these theoretical and experimental studies correspond to a pH of about 11 (pKa = 11.7 for H2O2), in which the reaction to 1O2 is only weakly driven. Conversely, 1O2 was not detected with alkyl hydroperoxides ROOH as the proton source (pKa ≈ 12.6) (ref. 45,48), a result that insufficient driving forces can now explain.
Relation between \({{\boldsymbol{E}}}_{{\genfrac{}{}{0ex}{}{1}{}{\bf{O}}}_{2}/{{\bf{O}}}_{{\bf{2}}}^{-}}^{\circ }\) and the 1O2 fraction
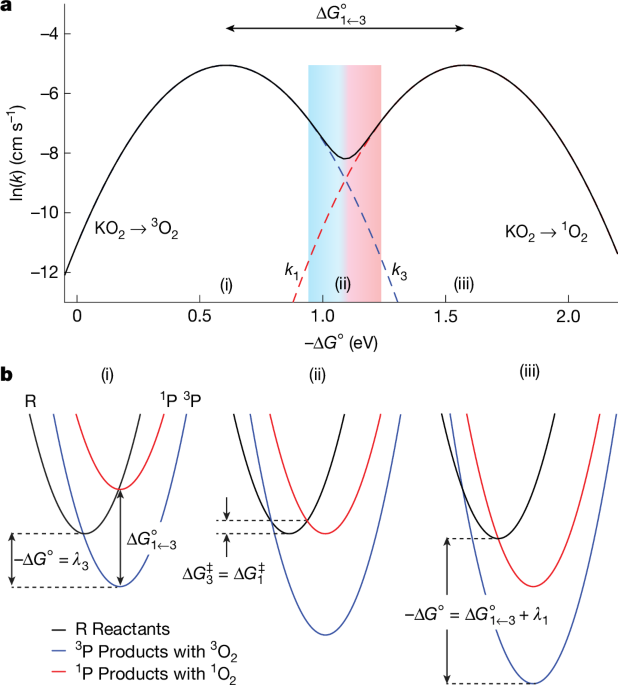
The electrochemiluminescence literature refers to energy-sufficient processes to form the electronically excited species23,28,49. For example, consider the generic redox couples R/R•– and M•+/M (note that these could be, for example, 3O2/O2− and RMox/RMred). The process R•– + M•+ → 1R* + M is considered energy-sufficient to form the excited species 1R*, if \(({E}_{{{\rm{M}}}^{\cdot +}/{\rm{M}}}^{\circ }-{E}_{{\rm{R}}/{{\rm{R}}}^{\cdot -}}^{\circ })F\ge \,-\Delta {H}^{\circ }(\,\genfrac{}{}{0ex}{}{1}{}\,{{\rm{R}}}^{* }\leftarrow {\rm{R}})\). This condition is fulfilled if the potential of the oxidizing redox couple exceeds the redox potential of the excited species: \({E}_{{{\rm{M}}}^{\cdot +}/{\rm{M}}}^{\circ }\ge {E}_{\genfrac{}{}{0ex}{}{1}{}{{\rm{R}}}^{* }/{{\rm{R}}}^{\cdot -}}^{\circ }={E}_{{{\rm{R}}/{\rm{R}}}^{\cdot -}}^{\circ }+\Delta {H}^{\circ }(\,\genfrac{}{}{0ex}{}{1}{}\,{{\rm{R}}}^{* }\leftarrow {\rm{R}})/F\). The connotation of energy-sufficient processes led to the interpretation that \({E}_{\genfrac{}{}{0ex}{}{1}{}{{\rm{R}}}^{* }/{{\rm{R}}}^{\cdot -}}^{\circ }\) or \({E}_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2}/{{\rm{O}}}_{2}^{-}}^{\circ }\) establishes a threshold above which the excited species rather than ground state species forms18,24,26. Extended Data Fig. 4a shows that reaching this threshold potential (or the driving force for which this is exceeded) gives no indication about the extent to which 1O2 rather than 3O2 forms. An onset of 1O2 may be expected at \({\Delta G}_{1\leftarrow 3}^{\circ }\), but its formation will become significant only for driving forces –ΔG° > λ3, for which 3O2 formation slows down to benefit 1O2 formation.
Thermodynamics in mixed alkali metal/TBA+ electrolytes
Superoxide disproportionation in Li+ and Na+ containing glyme electrolytes was found to always yield some 1O2 according to 2MO2 → M2O2 + x3O2 + (1 − x)1O2 (refs. 13,16,18,19). Li+ yielded small fractions (about 2%) at large kinetics, and Na+ yielded larger fractions (around 12%) at slow kinetics16. Often, electrolytes for non-aqueous metal-O2 batteries contain weakly Lewis acidic cations, such as tetrabutylammonium (TBA+) or other cations from ionic liquid electrolytes. In mixed Li+/TBA+ and Na+/TBA+ (1/1) electrolytes, the 1O2 yields increased to about 20 and 18%, respectively. The reasons for this behaviour must, hence, lie in (1) already sufficient driving forces for 1O2 formation in pure Li+ and Na+ electrolytes; and (2) increasing driving forces on adding TBA+.
M2O2 (M = Li+, Na+) are insoluble50,51 and the potential \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{{\rm{M}}}_{2}{{\rm{O}}}_{2}({\rm{s}})}^{\circ }\,=\) \({\Delta }_{{\rm{f}}}{G}^{\circ }({{\rm{M}}}_{2}{{\rm{O}}}_{2(\text{s})})/F=2.96\,{\rm{V}}\) compared with M+/M, therefore, fixed to the value obtained using the formation energy –ΔfG° of solid Li2O2(s). Given that the superoxide/M2O2(s) couple acts as the oxidant during superoxide disproportionation, the driving force is given by \(-\Delta G=({E}_{{\rm{superoxide}}/{{\rm{M}}}_{2}{{\rm{O}}}_{2(\text{s})}}-{E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{\rm{superoxide}}})F\). Note that here superoxide does not denote a particular species, but it could be anything, including solid MO2(s), solvated \({({{\rm{M}}}^{+}{{\rm{O}}}_{2}^{-})}_{n\ge 1,({\rm{sln}})}\) clusters and ion pairs, or the weakly coordinated \({({{\rm{TBA}}}^{+}{{\rm{O}}}_{2}^{-})}_{({\rm{sln}})}\). \({E}_{{\rm{superoxide}}/{\text{M}}_{2}{\text{O}}_{2(\text{s})}}\) cannot be directly measured but can be inferred from \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{{\rm{M}}}_{2}{{\rm{O}}}_{2(\text{s})}}\) and \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{\rm{superoxide}}}\). Using ΔG = –zFE, where z is the number of transferred electrons, it can be derived that \({E}_{{\rm{superoxide}}/{{\rm{M}}}_{2}{{\rm{O}}}_{2(\text{s})}}=2{E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{{\rm{M}}}_{2}{{\rm{O}}}_{2(\text{s})}}-{E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{\rm{superoxide}}}\). For the stable solid compounds (Li2O2, Na2O2, NaO2, K2O2 and KO2), tabulated formation energies ΔfG° can be found and the \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{{\rm{M}}}_{2}{{\rm{O}}}_{2}({\rm{s}})}^{\circ }\) and \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{\rm{M}}{{\rm{O}}}_{2}({\rm{s}})}^{\circ }\) be calculated as shown in Extended Data Fig. 5a. On the basis of this, KO2 is not expected to disproportionate to K2O2 and the K+-case, hence, not be further considered.
\({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{\rm{superoxide}}}\) require further consideration given the electrolyte-dependent solubilities of superoxide. Theoretical work shows that solvated \({({{\rm{Li}}}^{+}{{\rm{O}}}_{2}^{-})}_{n\ge 1,({\rm{sln}})}\) species are less stable in terms of Gibbs free energy than the bulk solid LiO2(s), but as the cluster size n grows, the structure approaches bulk MO2(s) and free energy approaches a constant value16,50,51. Aggregation into \({({{\rm{Li}}}^{+}{{\rm{O}}}_{2}^{-})}_{n > 1,({\rm{sln}})}\) clusters stabilizes the solvated species relative to separated \({({{\rm{Li}}}^{+}{{\rm{O}}}_{2}^{-})}_{({\rm{sln}})}\) species16,34. The Gibbs free energy grows, therefore, in the order of increasing solvation: LiO2(s) < (Li+O2–)n>1,(sln) < (Li+O2–)(sln). Accordingly, the potentials in pure M+ electrolyte are in the order and within the limits \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{{\rm{LiO}}}_{2(\text{s})}} > {E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{({{\rm{Li}}}^{+}{{\rm{O}}}_{2}^{-})}_{n > 1({\rm{sln}})}} > {E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{({{\rm{Li}}}^{+}{{\rm{O}}}_{2}^{-})}_{({\rm{sln}})}}\). If TBA+ is present, the even weaker association in \({({{\rm{TBA}}}^{+}{{\rm{O}}}_{2}^{-})}_{({\rm{sln}})}\) extends the lower potential limit to \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{({{\rm{TBA}}}^{+}{{\rm{O}}}_{2}^{-})}_{({\rm{sln}})}}\). The values for these potential limits, as shown in Fig. 3a, Extended Data Fig. 5c,d, were estimated from cyclic voltammograms with the salt shifting from pure M+ to pure TBA+ (Extended Data Fig. 5b). The largely solvation-independent DMFc/DMFc+ redox couple was used as internal standard. ΔfG° of solid LiO2(s) is not available, but \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{\rm{Li}}{{\rm{O}}}_{2}({\rm{s}})}\) may be estimated from cyclic voltammograms in poorly solvating electrolytes, in which large \({({{\rm{Li}}}^{+}{{\rm{O}}}_{2}^{-})}_{n,({\rm{sln}})}\) clusters approach the thermodynamics of LiO2(s). The shift in the onset of O2 reduction in Li+ compared with TBA+ electrolyte at slow scan rates was taken as the difference between \({E}_{{{}^{3}{\rm{O}}}_{2}/{{\rm{LiO}}}_{2(\text{s})}}\) and \({E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{({{\rm{TBA}}}^{+}{{\rm{O}}}_{2}^{-})}_{({\rm{sln}})}}.{E}_{{\genfrac{}{}{0ex}{}{3}{}{\rm{O}}}_{2}/{({{\rm{Li}}}^{+}{{\rm{O}}}_{2}^{-})}_{({\rm{sln}})}}\) as the lower limit of potentials in pure Li+ electrolyte was estimated from the potential shift between pure Li+ and pure TBA+ electrolytes with the highly solvating solvent 1-methylimidazole. These were taken from ref. 32 and show a shift of 33 mV.
Extended Data Fig. 5d shows the analogous thermodynamics for Na+/TBA+ mixtures as shown in Fig. 3 for Li+/TBA+. Considerations as above for the relative stabilities of the Li superoxides apply analogously to the relative stability of Na superoxide clusters compared with the NaO2(s) bulk. Theoretical work has similarly shown the stabilization by forming \({({{\rm{Na}}}^{+}{{{\rm{O}}}_{2}}^{-})}_{n > 1,({\rm{sln}})}\) clusters52,53. Extended Data Fig. 5e shows the NIR emission on adding KO2 into glyme electrolyte containing various Na+/TBA+ ratios. The data show that, even in a pure Na+ electrolyte, the driving force is sufficient for 1O2 formation and that added TBA+ increases the driving force and formation.
More generally, the electrolyte properties (solvent(s), salts(s) and their concentrations) will affect the reorganization energy and hence the maxima and crossing point of the two kinetic parabolas. The classical approach to accounting for this is the equation given by Marcus54, which connects the reorganization energy with the effective dielectric properties of the electrolyte and the separation of the redox centres. A lower dielectric constant and smaller separation will result in a larger reorganization energy. A refined equation by Marcus55 further takes into account the ionic environment. These considerations apply well, for example, to aqueous anionic redox couples and the series of alkali metal cations from Li+ to Cs+ as spectator cations, in which λ decreases56. However, caution is required with non-aqueous, low dielectric constant media, in which strong ion pairing occurs. Some works suggest an inverse trend for λ among the alkali metals19,57. Ion pairing and even clustering is particularly severe for (su)peroxide as the redox anions as discussed above. Superoxide forms in non-aqueous Li+ and Na+ electrolytes clusters32,33 and the peroxides are practically insoluble51. The order and extent to which the reorganization energy changes for superoxide oxidation in non-aqueous media among the alkali cations may, therefore, not be predicted straightforwardly and would merit further investigation. As we observe 1O2 at low driving force for the Na+ electrolyte, the reorganization energy appears sufficiently low therein.
Wider relevance for life sciences and energy
Our study contributes to understanding how the pH affects the link between the four important reactive oxygen species (ROS) superoxide, peroxide, 3O2 and 1O2. Disproportionation is notably the pathway to maintain a low superoxide concentration. However, detoxification from superoxide produces the harmful 1O2. Superoxide occurs in cells in several organelles with different pH levels between 4.7 and 8, but the superoxide-degrading enzyme superoxide dismutase occurs only in neutral to basic organelles35. In pathological situations, the pH balance is known to be affected (typically towards lower pH) and therefore signalling, redox regulation and defence58,59. Our study contributes to the understanding of how the redox chemistry of superoxide, pH and 1O2 formation are linked. We noted that in non-aqueous media, superoxide in contact with CO2 forms 1O2. Given the ubiquity of CO2 in organelles containing superoxide, further investigations into the aqueous chemistry of CO2 and superoxide are warranted.
For energy applications, further relevance and future research directions emerge: (1) For suppressing 1O2, generally, the driving force should be decreased, and the reorganization energy for the superoxide oxidation reaction should be increased. The classical equation by Marcus54, which connects the reorganization energy with the effective dielectric properties of the electrolyte and the separation of the redox centres, applies well to aqueous anionic redox couples56. For non-aqueous, low dielectric constant media, in which strong ion pairing occurs, particularly so with superoxide, the change in reorganization energy among different cations and electrolytes may not be predicted straightforwardly and would merit further investigation. (2) Oxygen evolution catalysis from water: metal-superoxo species have been identified as preceding the O2 evolution on, for example, the extensively studied Ni(Fe)OOH or CoOOH catalysts10. The metal Mn–1/n redox couple is considered to oxidize the superoxo moiety to O2 (refs. 10,60). Some of the most active Mn–1/n metal redox couples are typically a few 100 mV above the 1O2/superoxide potential shown in Fig. 4a and provide, in principle, enough driving force for 1O2 evolution. For example, at pH 14 \({E}_{{\genfrac{}{}{0ex}{}{1}{}{\rm{O}}}_{2}/{{{\rm{O}}}_{2}}^{-}}^{\circ }=1.32\,{\rm{V}}\), \({E}_{{{\rm{Co}}}^{{\rm{II}}/{\rm{III}}}}^{\circ }\approx 1.25\,{\rm{V}}\), \({E}_{{{\rm{Co}}}^{{\rm{III}}/{\rm{IV}}}}^{\circ }\approx 1.5\,{\rm{V}}\), \({E}_{{{\rm{Ni}}}^{{\rm{II}}/{\rm{III}}}}^{\circ }\approx 1.52\,{\rm{V}}\) and \({E}_{{{\rm{Ni}}}^{{\rm{III}}/{\rm{IV}}}}^{\circ }\approx 1.6\,{\rm{V}}\) on the RHE scale (refs. 10,60). Further investigations specifically on 1O2 evolution in oxygen evolution catalysis are therefore warranted. (3) Both Li-stoichiometric6 and Li-rich transition metal (TM, for example, Ni, Mn and Co) oxide2,7,61,62 intercalation materials used for positive electrodes in Li- or Na-ion batteries are known to undergo parasitic lattice oxygen loss at high states of charge. Both the intercalation material and the electrolyte degrade, hampering long-term cyclability. However, non-matching patterns of O2 and CO2/CO release from electrolyte decomposition (all containing lattice O as shown by isotopic labelling7,62) and enhanced lattice O loss with surface carbonates present7 remain unexplained and highlight the need for a deeper understanding of the prevailing ROS and decomposition pathways. For LiNiO2, 1O2 evolution has been suggested to result from the disproportionation of oxide radicals6. More generally, 1O2 may evolve from superoxo species (at the lattice surface, in (su)peroxocarbonates4,30) at the available driving forces. The oxidants could be a combination of (su)peroxides (for example, coordinated by TMs or carbonates) that get stabilized by further reduction and TM redox, such as CoIII/IV or NiIII/IV. Further investigations into the involvement of 1O2 evolution in TM oxide outgassing and surface reactions are therefore warranted.
The results expand the current knowledge on the electrogeneration of excited species more generally and pose open questions about the origin. Specifically, the process is more effective than typically assumed, given that \({\Delta G}_{1\leftarrow 3}^{\circ } < {\Delta H}_{1\leftarrow 3}^{\circ }\) and that Zel,1 ≫ Zel,3. Electrogeneration of excited ROS has significance ranging from biological systems to energy storage. Reactive excited species in life are very broadly associated with pathogenic events1,63. Recombination reactions in redox flow batteries are recognized to cause self-discharge, but this has so far not been recognized to potentially form extremely energetic excited species. Soluble parasitic oxidized and reduced species at the cathode and anode of Li- and Na-ion batteries may recombine to form energetic excited species.