Reagents were obtained from the following sources: antibodies to S6K pT389 (9234), S6K (2708), MIOS (13557), WDR59 (53385), Sestrin2 (8487), MYC epitope tag (2276 and 2278), Flag epitope tag (14793), HA epitope tag (3724) and horseradish peroxidase-linked anti-rabbit secondary antibody (7074) were from Cell Signaling Technology; antibodies to SEH1L (ab218531) and DEPDC5 (ab213181 and ab185565) were from Abcam; antibodies to SEC13 (15397-1-AP), WDR24 (20778-1-AP) and KPTN (16094-1-AP) were from Proteintech; antibodies to Raptor (09-217) and Flag-M2 (F1804) used for preparing anti-Flag magnetic beads were from MilliporeSigma; and antibody to NPRL3 was from Novus Biologicals (NBP1-88447). InstantBlue Coomassie Protein Stain was from Abcam; anti-Flag-M2 affinity gels, ATP and amino acids were from MilliporeSigma; DMEM, Expi293 Expression Medium, inactivated fetal serum, Dynabeads M-270 epoxy, Dynabeads protein G and anti-HA magnetic beads were from Thermo Fisher Scientific; XtremeGene9, PhosSTOP and cOmplete Protease Inhibitor were from Roche; amino acid-deficient RPMI (R8999-03A and R9010-01) was from US Biologicals; and PEI MAX 40 kDa was from Polysciences.
Cell lines and tissue culture
Adherent HEK293T cells were cultured in DMEM (Thermo Fisher Scientific) with 10% inactivated fetal serum (Thermo Fisher Scientific) and 4.5 g l−1 glucose containing 2 mM GlutaMAX (Thermo Fisher Scientific), 100 IU ml−1 penicillin and 100 µg ml−1 streptomycin. Adherent cell lines were maintained at 37 °C and 5% CO2. Expi293F cells were grown in Expi293 medium (Thermo Fisher Scientific) supplemented with 100 IU ml−1 penicillin and 100 µg ml−1 streptomycin. Suspension cells were grown in an INFORS Multitron Pro shaker operating at 37 °C, 8% CO2, 80% humidity and 90–125 rpm. HEK293T cells were obtained from the American Type Culture Collection, and Expi293F cells were obtained from Thermo Fisher Scientific. All cell lines were validated and tested for mycoplasma.
Transfections, cell lysis and immunoprecipitation experiments
Transfection, cell lysis and immunoprecipitations were performed as previously described10. To harvest samples, cells were washed once with ice-cold PBS and then lysed with lysis buffer (1% Triton X-100, 40 mM HEPES pH 7.4, 10 mM β-glycerol phosphate, 10 mM pyrophosphate and 2.5 mM MgCl2) and 1 tablet of EDTA-free protease cocktail (Roche) per 25 ml buffer. Cell lysates were clarified by centrifugation at 21,000g at 4 °C for 10 min.
For anti-Flag immunoprecipitations, magnetic beads bound to antibody recognizing the Flag epitope tag were prepared in-house by coupling Dynabeads M-270 epoxy (Thermo Fisher Scientific) to Flag-M2 antibody (MilliporeSigma) as previously described10. For anti-HA immunoprecipitations, anti-HA-coupled magnetic beads (Thermo Fisher Scientific) were used. Before use, beads were washed three times with Triton X-100 lysis buffer and then incubated with the supernatant of each clarified lysate for 1 h at 4 °C. Each immunoprecipitation used 10 µl of anti-Flag or 30 µl of anti-HA magnetic beads. For anti-MYC immunoprecipitations (Extended Data Fig. 9b), 4 µl of anti-MYC antibody was added to each sample of clarified lysate and incubated at 4 °C for 1 h. Subsequently, 30 µl of pre-washed protein G magnetic beads was added to each sample and incubated for an additional 1 h at 4 °C. Following immunoprecipitation, beads were washed one time with Triton X-100 lysis buffer and two times with Triton X-100 lysis buffer supplemented to contain 150–500 mM NaCl. Immunoprecipitated proteins were denatured by addition of SDS–PAGE sample buffer and boiling for 5 min at 95 °C. Immunoprecipitated proteins were then resolved by 4–20% SDS–PAGE before analysis by immunoblotting.
For experiments requiring transfection of cDNAs into HEK293T cells, 2 × 106 cells were plated in 10-cm culture dishes or 5 × 106 cells in 15-cm culture dishes. Twenty-four hours later, cells were transfected with the appropriate pRK5-based cDNA expression plasmids using the polyethylenimine method, as previously described30. The total amount of plasmid DNA in each transfection was normalized to 5 μg with empty pRK5 (10-cm plates) or 20 µg of empty pRK5 (15-cm plates). Thirty-six hours following transfection, cells were lysed as described above.
For experiments that required amino acid starvation, cells were incubated in amino acid-free RPMI for 60 min, as previously described31. To restimulate cells following starvation, an amino acid mixture prepared from individual powders of amino acids (MilliporeSigma) was added to cell culture media for 15 min. Starvations for individual amino acids were performed similarly.
Experiments evaluating the in vitro dissociation of GATOR2–sensors complexes were performed as previously described16,17,19,21, with the following modifications. Following immunoprecipitation, washing and equilibration in cytosolic buffer, the beads incubated with either 300 µM leucine or 400 µM arginine for 20 min at 4 °C with gentle rocking. The beads were then washed twice more, after which the samples were denatured by addition of sample buffer and analysed by immunoblotting, as described above.
Protein expression and purification
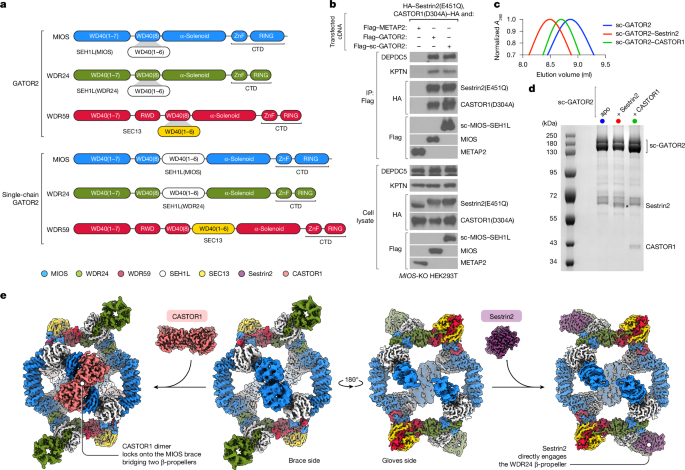
Sestrin2, CASTOR1 and wild-type GATOR2 were expressed and purified as previously described10. For purification of sc-GATOR2, 5 l of Expi293F cells grown in Expi293 medium (Thermo Fisher Scientific) was transiently transfected with cDNAs encoding N-terminally Flag-tagged sc-WDR59–SEC13 (Addgene 237416), tag-free sc-MIOS–SEH1L (Addgene 237417) and tag-free sc-WDR24–SEH1L (Addgene 237418) in the pRK5 vector. To prepare sc-GATOR2 in complex with Sestrin2 or CASTOR1, the cDNA mixture was supplemented with HA–Sestrin2(E451Q) (Addgene 237420) or HA–CASTOR1(D304A) (Addgene 237419) in the pRK5 vector, respectively. Cells were transfected at a density of 3 × 106 per millilitre with 1 mg total cDNA and 4 mg PEI MAX 40 K (Polysciences) per litre culture. Seventy-two hours after transfection, cells were harvested, washed in ice-cold PBS and then lysed in Triton lysis buffer (TLB; 1% Triton X-100, 50 mM HEPES pH 7.4, 100 mM NaCl, 50 mM arginine, 50 mM glutamic acid and 2.5 mM MgCl2) with EDTA-free protease inhibitor cocktail (Roche; 1 tablet per 25 ml buffer) and 1 mM ATP (100 ml of lysis buffer was used per litre cell culture). The lysate was cleared by ultracentrifugation at 50,000g for 20 min. Pre-washed anti-FLAG-M2 affinity gel (MilliporeSigma) was added to the clarified lysate (2 ml slurry per litre culture) and incubated for 2 h at 4 °C on a nutator. The beads were washed once in TLB, twice in TLB supplemented with 50 mM NaCl, once in CHAPS buffer (0.1% CHAPS, 50 mM HEPES pH 7.4, 150 mM NaCl, 50 mM arginine, 50 mM glutamic acid and 2 mM MgCl2), once in CHAPS buffer supplemented with 20 mM MgCl2 and 5 mM ATP, and once again in CHAPS buffer. The GATOR2 complex was eluted from the beads by competitive elution in CHAPS buffer supplemented with 0.5 mg ml−1 3× Flag peptide (sequence DYKDHDGDYKDHDIDYKDDDDK). Eluate was collected with bead separator columns, concentrated with a regenerated-cellulose centrifugal filter (100 kDa MWCO; MilliporeSigma), and clarified by centrifugation at 21,000g for 10 min at 4 °C to remove any debris. Further purification was performed by size-exclusion chromatography (SEC) using a TSKgel G4000SWxl column (Tosoh) pre-equilibrated in CHAPS buffer supplemented with 2 mM dithiothreitol. Before SEC purification of the sc-GATOR2–Sestrin2 or sc-GATOR2–CASTOR1 complexes, the eluates were supplemented with fivefold molar excess of purified wild-type Sestrin2 or CASTOR1(D304A), respectively. Elution fractions were resolved by SDS–PAGE (4–12% Bolt gels, Thermo Fisher Scientific) and stained with InstantBlue Coomassie Protein Stain (Abcam). Pure protein fractions were pooled, concentrated by centrifugal ultrafiltration, supplemented with 10% glycerol and snap frozen in liquid nitrogen before storage at −80 °C. For analysis by cryo-EM, GATOR2 samples were prepared immediately following SEC. Immediately before grid preparation of the sc-GATOR2–Sestrin2 or sc-GATOR2–CASTOR1 complexes, the purified samples were supplemented with threefold molar excess of purified wild-type Sestrin2 or CASTOR1(D304A), respectively.
cDNA cloning
To generate single-chain constructs, fragments with the following boundaries were prepared by PCR amplification using Q5 High-Fidelity Polymerase (New England Biolabs) and assembled into pRK5 by Gibson Assembly (New England Biolabs):
-
sc-WDR24–SEH1L: WDR24(1–404)–SEH1L(1–360)–WDR24(405–790)
-
sc-MIOS–SEH1L: MIOS(1–380)–SEH1L(1–360)–MIOS(381–875)
-
sc-WDR59–SEC13: WDR59(1–651)–GSGSG–SEC13(1–321)–GSGSG–WDR59(652–974).
To generate point mutants in cDNAs for structural validation, site-directed mutagenesis was performed using the KLD Enzyme Mix (New England Biolabs) according to the manufacturer’s instructions.
Generation of cells stably expressing cDNAs
Cells that stably expressed cDNAs were generated as previously described8. The lentiviral expression plasmids used were: pLJM60-Flag-METAP2 and pLJM1-WDR24, with the latter containing site-directed mutations.
Molecular dynamics simulations
Molecular dynamics simulations were performed using GROMACS (2024-rc)32. The initial model was constructed using the coordinates of dimeric CASTOR1 from our cryo-EM structure of sc-GATOR2–CASTOR1, with an arginine ligand positioned in the binding pocket according to its placement in the crystal structure of arginine-bound CASTOR1 (PDB ID: 5I2C)19. To accurately quantify the relocation of the arginine-binding loop relative to the initial input state, simulations specifically targeting loop dynamics were conducted using a single CASTOR1 protomer. By contrast, simulations investigating the mechanism of arginine release and the swimming molecular dynamics binding study were performed using the CASTOR1 dimer to account for potential allosteric effects. Seven independent unbinding events were analysed, five of which exhibited the transient state described in the article. The swimming molecular dynamics simulation exposed the apo CASTOR1 dimer to 150 mM arginine.
Missing atoms were added, and protonation states of titratable residues were adjusted to reflect physiological pH using the Protein Repair and Analysis Server33. Protein interactions were modelled using the CHARMM36m force field34, and the solvent was represented explicitly using the TIP3P water model35. Protein chain termini were modelled in their charged ionic states. The arginine ligand topology was generated using ACPYPE (antexhamber Python parser interface)36. The system was solvated in a triclinic box of explicit TIP3P water molecules, with a 20 Å buffer between the protein and box edges. Na+ and Cl− ions were added to neutralize the system and achieve a physiological ionic strength of 150 mM.
Energy minimization was performed using the steepest descent algorithm until the maximum force on any atom fell below 1,000 kJ mol nm−1, ensuring the absence of steric clashes or inappropriate geometry. The system was then equilibrated in two stages: first, isothermal-isochoric (NVT) equilibration at 300 K for 100 ps using the V-rescale thermostat with a time constant of 0.1 ps; second, isothermal-isobaric (NPT) equilibration at 1 bar for 1 ns using the Parrinello–Rahman barostat with a time constant of 2.0 ps.
Production molecular dynamics simulations were typically run for 1,000 ns (2-fs time step) at 300 K and 1 bar. Periodic boundary conditions were applied in all three dimensions. Non-bonded interactions were calculated using a 1.0-nm cut-off for van der Waals forces, and long-range electrostatics were computed using the particle-mesh Ewald summation method with a Fourier spacing of 0.12 nm. Bonds involving hydrogen atoms were constrained using the LINCS algorithm. Simulations were run on the Stanford University high-performance computing cluster (SHERLOCK), utilizing 1× GPU and 4× CPU per simulation.
Trajectory analyses of RMSD were performed using the RMSD Trajectory Tool in VMD37. RMSD calculations assessed the stability and flexibility of the arginine-binding loop (CASTOR1 residues 273–277) by aligning the protein backbone to the average reference structure derived from aligned simulation frames.
In accordance with the reliability and reproducibility standards for molecular dynamics simulations for Nature38, additional details have been provided in Supplementary Table 4. Coordinate files corresponding to critical simulation outputs (Extended Data Fig. 7c–e) are available as Supplementary Information 1 and 2.
Cryo-EM grid preparation and data collection
Cryo-EM specimens were prepared using a Vitrobot Mark IV (Thermo Fisher Scientific). All three purified sc-GATOR2 complexes — apo state, Sestrin2 bound and CASTOR1 bound — were concentrated to 3 mg ml−1 in a final volume of 3 µl. These samples were applied to glow-discharged gold 300 square-mesh Quantifoil R 1.2/1.3 holey carbon grids (Quantifoil) and blotted from both sides for 3 s at 95% humidity before plunge freezing in liquid ethane.
Datasets were collected on a Titan Krios G3 electron microscope (Thermo Fisher Scientific) operated at 300 kV, equipped with a Gatan K3 direct detection camera (super-resolution counting mode) and a BioQuantum energy filters (slit width of 20 eV). Specific microscope parameters for each dataset are provided in Supplementary Tables 1–3. Fully automated data collection was performed using the EPU software.
Cryo-EM image processing
Data processing workflows for reconstitution of the three sc-GATOR2 complexes are illustrated in Supplementary Figs. 2–13. Details for each dataset are summarized below.
Large movie datasets recorded with a Titan Krios microscopes (27,853 for apo, 34,122 for Sestrin2 and 23,777 for CASTOR1) were corrected for drift using MotionCor2 implementation in RELION (v5.0)39,40,41. Contrast transfer function (CTF) parameters were determined using CTFFIND (v4.1.14)42. Motion-corrected micrographs were denoised using Topaz-Denoise43 with pretrained models. For each dataset, particle picking was performed in Topaz (v0.2.5)44 using two distinct search models: (1) a default pre-calculated model provided by the software developers, and (2) a custom-trained model generated from a manually picked subset of 1,000 GATOR2 particles. The resulting particle sets (2,515,780 and 2,512,791 for apo, 2,284,441 and 1,716,534 for Sestrin2 and 2,225,804 and 1,981,836 for CASTOR1) were extracted and downscaled for further processing in RELION.
Reference-based 2D classifications45 were applied to effectively eliminate contaminating non-GATOR2 particles and partially disassembled GATOR2 complexes. The deposited global consensus map from our previously published apo GATOR2 state (EMD-26519) served as the reference input map10. Remaining particles were reclassified in 3D, and the best classes from different Topaz sets (default and trained) were combined using a strict distance cut-off (150 Å) to remove duplicates. The resulting cleaned particle sets (758,113 for apo, 287,922 for Sestrin2 and 537,062 for CASTOR1) were then used to train a refined Topaz search model for a final round of reference-based particle picking. The collected data included a minor subset of CCT–TRiC complex particles, which co-eluted with GATOR2 during SEC due to their similar size of approximately 1 MDa. These particles were identified and removed during the initial 2D classification of each respective dataset.
The resulting refined particle sets (3,252,339 for apo, 2,987,526 for Sestrin2 and 2,418,964 for CASTOR1) were screened for intact sc-GATOR2 complex particles by reference-based 2D classifications (2,063,260 for apo, 1,240,221 for Sestrin2 and 1,248,533 for CASTOR1) and subsequent 3D classifications (806,219 for apo, 478,005 for Sestrin2 and 704,956 for CASTOR1) in RELION. The resulting cleaned refined particle sets were then merged with the clean default and trained sets, using a strict distance cut-off (150 Å) to remove duplicates. These combined particle sets (1,148,926 for apo, 605,308 for Sestrin2 bound and 865,410 for CASTOR1 bound) were re-extracted at full size and refined through iterative cycles of CTF and aberration refinement in RELION (per-particle defocus, per-micrograph astigmatism, beam tilt and higher-order aberrations)46. Alignment of the GATOR2 maps to C2 symmetry and correction of per-particle motion in RELION40 further improved the resolution of the reconstructions. These particle sets were then transferred to cryoSPARC (v4.5)47 and refined using iterative cycles of non-uniform refinement48 and per-particle CTF refinement46. The final consensus maps, generated with C2 symmetry applied, reached resolutions of 3.47 Å (apo state), 3.36 Å (Sestrin2-bound state) and 3.40 Å (CASTOR1-bound state), as determined by Fourier shell correlation at the 0.143 threshold between independently refined half-maps.
All sc-GATOR2 particle sets exhibited significant heterogeneity. In contrast to our previous GATOR2 dataset (EMD-26519), in which subunit dissociation complicated the analysis of compositional and conformational heterogeneity10, the newly designed single-chain constructs guaranteed compositional homogeneity. This allowed us to specifically focus on characterizing the intrinsic conformational flexibility of the GATOR2 complex. All particle sets were expanded using C2 symmetry (2,297,852 for apo, 1,210,616 for Sestrin2 and 1,730,820 for CASTOR1) and subjected to masking and local refinement in cryoSPARC (without particle subtraction). This allowed improved visualization of multiple overlapping regions within the GATOR2 complex: four regions for sc-GATOR2–Sestrin2 and five regions each for apo sc-GATOR2 and sc-GATOR2–CASTOR1 (Supplementary Figs. 2, 6 and 10). The resulting particles were further analysed for heterogeneity using 3D variability analysis (3DVA)49 in cryoSPARC (filter resolution = 5 Å and iterations = 40) to isolate particle subsets with the highest occupancy in each masked region and to identify rigid sections that move as semi-independent bodies.
These selected subsets underwent additional local refinement in cryoSPARC, followed by final sharpening using automated post-processing routines in DeepEMhancer (v0.16)50. High-resolution features were enhanced using the high-res algorithm (resolution cut-off = 2.5 Å), whereas low-resolution features were improved using the wide-target algorithm (resolution cut-off = 4.5 Å). Finally, high-occupancy composite maps (both unsharpened and DeepEMhancer sharpened) were generated in phenix.combine_focused_maps (Phenix v2.0)51 by combining rigid bodies within the respective sc-GATOR2 complexes (Supplementary Figs. 4, 8 and 12). The resulting composite sc-GATOR2 maps were generated as pseudosymmetric, with C2 symmetry applied to all structural regions except the brace MIOS–SEH1L β-propellers, which directly span the C2 symmetry axis. Because these β-propellers adopt two distinct orientations — one symmetric and one asymmetric (Supplementary Fig. 15) — we treated them as a C1 feature for consistency.
Model building and refinement
All model building tasks were performed using the C2-symmetric composite maps of the respective sc-GATOR2 complexes. Previously published structures — human apo GATOR2 (PDB ID: 7UHY)10, human leucine-bound Sestrin2 (PDB ID: 5DJ4)21 and human arginine-bound CASTOR1 (PDB ID: 5I2C)19 — were docked into the cryo-EM reconstructions using UCSF ChimeraX (v1.8)52. These initial models were iteratively rebuilt through cycles of interactive adjustments in Coot (v0.9.8)53 and refinement in phenix.real_space_refine (Phenix v2.0)54, incorporating AlphaFold 2 (refs. 55,56) predictions to improve backbone geometry in regions of lower map resolution. For each sc-GATOR2 complex, only one protomer (the asymmetric unit) was built and refined as described above; the full complex was generated by applying C2 symmetry operators. Model refinement was carried out with restraints applied to secondary structure elements and zinc-coordinating residues. Model quality was evaluated using MolProbity (v4.5.2)57.
The final models consist of the following: apo sc-GATOR2 (7,588 amino acids and 32 zinc ions), Sestrin2-bound sc-GATOR2 (8,426 amino acids and 32 zinc ions) and CASTOR1-bound sc-GATOR2 (9,594 amino acids and 30 zinc ions). The atomic model of leucine-bound Sestrin2 (originally PDB ID: 5DJ4) was rebuilt based on insights from the sc-GATOR2–Sestrin2 cryo-EM structure. Specifically, residues previously assigned as linker residues 233–240 were reassigned to residues 40–57 of the N-terminal domain (helix αN0). In addition, residues 249–255, 270–280 and 296–297 (the αL1–linker–αL2 segment) were added to the model based on weaker but interpretable electron density. The updated model was improved through iterative cycles of interactive model building in Coot and refinement using phenix.refine (Phenix v2.0)58. After each refinement cycle, the model was evaluated, geometry corrected and adjusted for density fit until convergence was reached. The final updated leucine-bound Sestrin2 model was deposited in the PDB under the accession code 9PDM.
Similarly, the atomic model of arginine-bound CASTOR1 (originally PDB ID: 5I2C) was rebuilt using insights from the sc-GATOR2–CASTOR1 cryo-EM structure. Residues 82–89, corresponding to the release loop, were added to the model based on weaker but interpretable electron density, and the model was iteratively refined as described above. The final updated arginine-bound CASTOR1 model was deposited in the PDB under accession code 9PDO.
Flexibility analysis of GATOR2 WDR24 β-propeller
To evaluate the effects of inhibitor (CASTOR1 and Sestrin2) binding on the dynamic behaviour of the WDR24 β-propeller, particle subsets with full inhibitor occupancy were first selected. Non-symmetry-expanded particle sets for sc-GATOR2–Sestrin2 (605,308 particles) and sc-GATOR2–CASTOR1 (865,410 particles) were analysed for heterogeneity using cryoSPARC 3DVA47,49 (filter resolution = 5 Å and iterations = 40). Masks were applied to cover Sestrin2, SEH1L(WDR24) and the WDR24 β-propeller for sc-GATOR2–Sestrin2, and the brace-associated CASTOR1, SEH1L(MIOS) and the MIOS β-propeller for sc-GATOR2–CASTOR1. The resulting particle subsets with full inhibitor occupancy (307,953 for sc-GATOR2–Sestrin2 and 559,052 for sc-GATOR2–CASTOR1) were locally refined using relaxed C2 symmetry parameters and the masks from 3DVA. To evaluate the flexibility of the WDR24 β-propeller, 3D classifications were performed using a global mask covering the entire GATOR2 complex and a targeted mask focusing on the two WDR24 β-propellers (Extended Data Fig. 9a). Hard classification was enforced (convergence criterion = 1% and filter resolution = 10 Å) to strictly distinguish between the presence or absence of the WDR24 β-propeller. Initial testing confirmed that 15,000 particles are sufficient to generate a high-quality sc-GATOR2 map. To ensure robustness and reproducibility, particles were repeatedly classified into subsets of 20,000, 22,000, 25,000, 28,000 and 30,000 particles per class. The resulting classes were categorized based on the presence of double, single or no WDR24 β-propellers on the GATOR2 complex surface (Extended Data Fig. 9a). Occupancy ratios were calculated and plotted, with error bars representing the range of values obtained across repeated classifications for each GATOR2 complex. The same WDR24 β-propeller masks and classification protocols were applied to the control particle set of apo sc-GATOR2 (1,148,926 particles).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.