Flies
Flies (D. melanogaster) were reared on standard cornmeal food at 25 °C and 60% humidity under a 12 h:12 h light:dark regimen. Details of genotype (including driver lines46) and source of flies are given in Supplementary Table 3. All experiments were performed in accordance with relevant guidelines and regulations. Animal sex is indicated below.
Imaging of neural activity
For voltage and Ca2+ imaging experiments we generally used 3- to 10-day-old female flies and performed either whole-brain explant dissections (ex vivo) or in vivo experiments as previously described3,15. If not indicated otherwise, morning experiments were performed at ZT (Zeitgeber time) 2–4 (during a 12:12 h light:dark cycle the onset of light is at ZT 0 and the offset of light is at ZT 12), midday experiments were performed at ZT 5–8 and night experiments were performed at ZT 14–18. For sleep deprivation, vials containing flies were fixed to an analogue Multi-Tube Vortexer (VX-2500, VWR) controlled by TriKinetics acquisition software. For sleep deprivation we applied a mechanical stimulus that lasted for 2 s randomized within a time window of 20 s 12–14 h prior to the experiment. For DD experiments, flies were kept in darkness for 24 h prior to performing the experiment. For all DD experiments (control and per mutants) we used 3–10 day old male flies because the per gene is located on the X chromosome and male flies therefore allow investigation of the null-mutant with only one mutated per allele26.
To record spontaneous activity in vivo, we immobilized the flies (except for Extended Data Fig. 5e, for which we did not fix the legs to quantify leg movement). As previous recordings have shown3 that the haemolymph contains around 20 mM of Mg2+, we used a high-Mg2+ external solution consisting of (in mM) 70 NaCl, 3 KCl, 1.5 CaCl2, 20 MgCl2, 1 NaH2PO4, 10 glucose, 10 sucrose, 8 trehalose, 5 TES (N-[tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid) and 26 NaHCO3. Please note that the observed coherent oscillations are comparable at low extracellular Mg2+ (5 mM; Extended Data Fig. 11d–f). External solution was adjusted to a pH of 7.4, with an osmolarity of 280 mmol kg−1. To reduce brain movement the external solution supplemented with 10% papain (Roche) was applied to the head capsule for 7 min and subsequently washed out. To block ionotropic GABA receptors, we applied 200 µM of picrotoxin (Tocris) to the head capsule. For chemogenetic inhibition in Extended Data Fig. 10d–h, we ectopically expressed histamine receptors (LexAop-ort)40,41 and added histamine (2 mM, Sigma-Aldrich) for 10 min before performing the ‘after’ measurement. The time window for measuring spontaneous activity was the same as for previous experiments.
To facilitate spontaneous activity ex vivo we used a low Mg2+ external solution (5 mM) consisting of (in mM) 90 NaCl, 3 KCl, 1.5 CaCl2, 5 MgCl2, 1 NaH2PO4, 10 glucose, 10 sucrose, 8 trehalose, 5 TES and 26 NaHCO3. To compensate for Cl−, we adjusted NaCl concentration in the external solution, which did not alter spontaneous activity3. The high-Mg2+ external solution (20 mM) was used in ex vivo optogenetic experiments to suppress spontaneous activity. High external Mg2+ suppresses spontaneous activity through surface charge screening effects47 and by limiting Ca2+ influx for synaptic transmission48. Strong optogenetic stimulation of all neurons within a neural population is sufficient to overcome this limiting effect and induce synaptic transmission.
Imaging was performed using an Olympus BX51WI microscope using a Plan Apochromat 40×, numerical aperture 0.8, water-immersion objective (Olympus). The objective C-mount image was projected either onto an Andor iXon-888 CCD camera controlled by Andor Solis software or onto a Kinetix22 sCMOS camera (Teledyne Photometrics) controlled by micromanager. Voltage imaging was performed at 80–100 frames per second, whereas Ca2+ imaging was performed at ~20 frames per second. In general, recordings lasted for 30–40 s but were also extended to 120 s with a recording speed of 20 Hz to demonstrate the stability of oscillatory activity (Extended Data Fig. 11a–c). ArcLight and GCaMP were excited at 475/35 nm using a Lumencor Spectra X-Light engine (Lumencor) LED system. For Ca2+ imaging we used either GCaMP6f or GCaMP7b. The increased brightness and sensitivity of GCaMP7b allows improved detection of anatomical structures and neural activity in promotor lines with low baseline expression49. To avoid saturated fluorescent images, we adjusted the LED power for each recording individually. To avoid a potential influence of light on the animal’s internal state, an aperture was used to minimize the diameter of the LED light. To determine absolute fluorescence in single helicon cell bodies (Extended Data Fig. 6d), LED power was kept constant at 24% throughout all recordings. For dual-colour voltage imaging, ArcLight and Varnam were simultaneously excited at 475/35 nm and 542/33 nm using a Spectra X-Light engine LED (Lumencor) system. For separating the emission wavelengths, we used the W-View Gemini image splitter (Hamamatsu) with an adequate dichroic mirror for ArcLight (520/35 nm) and Varnam (641/75 nm). The magnitude of relative changes in fluorescence between different networks is derived from the properties of the different voltage indicators, as well as from the number of cells comprised in the network. For visual stimulation in Fig. 5 and Extended Data Fig. 10, we used a 637/12 nm LED at 20% (190–300 µW cm−2) and an aperture build in the LED pathway to switch between focal optogenetic and broad visual stimulation.
For dual-colour voltage imaging and for optogenetic experiments, all-trans retinal (Sigma-Aldrich) was added to the fly food as a 50 mM stock dissolved in 95 % ethanol. Flies were collected after eclosion and transferred to fly food containing 1.3 mM of all-trans retinal for 2–4 days. Unless indicated otherwise, CsChrimson was excited continuously at 637/12 nm at a power density of 190–740 µW cm−2. For optogenetic hyperpolarization GtACR1 was excited at 542/33 nm at a similar power density. Due to potential spectral overlap, excitation of ArcLight and GCaMP at 475/35 nm was kept minimal (20–170 µW cm−2). To reduce optical artefacts generated by red light stimulation we used a custom-made dual-band beamsplitter filter cube allowing red light to pass the excitation filter but not the emission filter. To further reduce optical artefacts, we performed a background subtraction on imaging recordings (see quantification and statistical analysis).
Leg movement in Extended Data Fig. 5e was quantified using Python (3.11.0) and OpenCV (4.9.0) as previously described21. Fly behaviour was recorded during voltage imaging using a Basler acA1440-220um camera at a frame rate of 25 frames per second. To synchronize this video with the in vivo recording, start and end of the recording were identified on the basis of changes in illumination patterns. Data from 2 to 28 s (650 frames) were analysed. For each frame, a region of interest was created (custom made for each fly) that mainly focused on the body of the fly and its legs and clipped the frame. Images were converted from BGR to grayscale. The absolute difference of a current frame with the previous one (difference frame) was computed and thresholded. To fill holes, this binarized image was dilated. Contours were identified and selected for a specific area threshold. Finally, rectangles were drawn over the contours to manually verify the movement patterns. When a specific frame had contours above the set threshold it was regarded as ‘moved’ and otherwise it was classed as ‘still’. For every fly the percentage of frames ‘moved’ was computed. For comparing movement intensity with correlation coefficients of electrical patterns, we identified specific bouts of movement and non-movement (on the few seconds range) on the basis of smoothed activity profiles across time. Each frame within a bout was categorized as either ‘moved’ = 1 or ‘not moved’ = 0 and all values across an identified bout were averaged. Movement data was compared with the electrical correlation coefficient measured for the respective bout.
Single-fly tracking experiments
For measuring locomotor activity, we used female flies that were collected within 2–3 days after eclosion. Experiments were performed in the dark in circular arenas (2.5 cm diameter) illuminated from below using a low power infrared (850 nm) LED panel (2.7 mW cm−2 at 520 nm). Flies were recorded from above at 15 frames per second using the high-resolution camera Flea3 USB3 (FL3-U3-13E4C-C, Teledyne FLIR) attached to a 25 mm focal length lens (63-246, Edmund Optics) and an infrared filter (10000314, Neewer) as described50. Camera control was performed using Point Grey FlyCap2 software (version 2.10.3.266). For optogenetic experiments (Figs. 2 and 4 and Extended Data Figs. 3, 8 and 9), flies were reared for 2–4 days after collection on food supplemented with 1.3 mM all-trans retinal. To avoid optogenetic activation during this time, vials containing flies were partially covered with a foil, which still allowed them to perceive background light. All experiments were carried out at room temperature and during the subjective day of the fly (from ZT 1–10).
At the start of each experiment, flies were allowed to acclimatize for 10 min. We then measured the flies’ initial baseline locomotor activity for 5 min (see Fig. 2d for an example). For optogenetic activation experiments, flies were then exposed to 5 min of 5-ms pulses of red light (~20 mW cm−2 at 632 nm) from below at a specific frequency indicated in the figure legends. Subsequently locomotor activity was recorded for another 10 min. For optogenetic hyperpolarization of helicon cells (Extended Data Fig. 8h,i), flies were exposed to 5 ms pulses of green light from below (~3.3 mW cm−2 at 520 nm) at 1 Hz for 5 min.
For measuring absolute locomotor activity (distance travelled) and mean velocities, we considered all movements. To determine whether flies were perturbed in their ability to walk, we only considered velocities equal or higher than 0.25 cm s−1 (walking velocity and percentage of time walking)5,36. In order to normalize locomotor variables to the baseline activity, we computed the effect of the optogenetic protocol on baseline locomotion—that is, effect on total distance = (distance travelled during stimulation – distance travelled during baseline)/(distance travelled during stimulation + distance travelled during baseline). To evaluate turning behaviour (Extended Data Fig. 9), we simplified flies’ trajectories using an implementation of the Ramer–Douglas–Peucker algorithm (https://github.com/sebleier/RDP/), computed the direction vectors of the simplified trajectories and classified changes in direction equal or higher than 47° as turns. For high resolution analysis of behaviour during R5 activation experiments (Fig. 2h), we manually annotated the occurrence of different behaviours: walking, grooming and resting (flies are immobile with an upright body posture; see Supplementary Video 1).
For the analysis of resting activity in sleep-deprived flies (Extended Data Fig. 3k,l), we quantified the occurrence of rest bouts (periods of duration ranging from 10 s to 5 min with a velocity smaller than 0.25 cm s−1) during a 10 min baseline. The resting activity of flies that remained awake throughout this period (‘no sleep’) was compared to that of flies that were awake at the beginning of the experiment and later met the criteria for sleep (at least 5 min with a velocity smaller than 0.25 cm s−1). In flies that fell asleep, their resting activity was analysed in the period before falling asleep (‘before sleep’). The mean duration and frequency of resting bouts, as well as the percentage of time spent resting of each fly were normalized to the time that a fly spent awake during the 10 min baseline.
Behavioural responsiveness
To test visual and mechanical responsiveness of sleep-deprived flies (‘quiescent’; Fig. 4g,h and Extended Data Fig. 8a–f) vials containing flies reared in retinal food for 2–3 days were fixed to an analogue Multi-Tube Vortexer (VX-2500, VWR) controlled by TriKinetics acquisition software. For sleep deprivation, a mechanical stimulus lasting 2 s was provided randomly within a time window of 20 s from the beginning of the subjective night (ZT 12) to the start of the experiment (ZT 1–7). Only sleep-deprived flies that did not move in the last 5 min of recording baseline activity (meeting the generally accepted criteria for sleep) were included in the analysis. To assess visual responsiveness during optogenetic activation (Fig. 4g, h and Extended Data Fig. 8a–c) flies were exposed to a 1-min green light stimulus from above (0.37 mW cm−2 measured at 520 nm). Responsiveness to light was evaluated by measuring changes in a fly’s velocity (‘effect on mean velocity’). To determine whether flies were awakened by light, we only considered movements equal or higher than 0.25 cm s−1.
To test responsiveness to an air puff during optogenetic activation in rested and sleep-deprived flies (that were quiescent, Fig. 2j and Extended Data Fig. 8d–f), flies were individually loaded in Trikinetics glass tubes (5 × 65 mm) connected to an air pump on one side and sealed with perforated parafilm on the other end to allow air flow. Tubes were attached to the LED panel for 1 Hz optogenetic activation and exposed to a constant 10 s air puff (~10 l min−1) that was sufficiently strong to startle the flies. Responsiveness was measured by comparing changes in mean velocity before and after the air puff (40 s each). To exclude movements during the air puff, data 5 s before and after stimulus were not included in the analysis. Only sleep-deprived flies that did not move in the last 5 min of recording baseline activity (meeting the generally accepted criteria for sleep) were included in the analysis.
Patch clamp recordings
For optogenetic experiments, 1- to 2-day-old female flies were reared for 2 days on food supplemented with 1.3 mM all-trans retinal. In vivo whole-cell patch clamp experiments were performed from GFP labelled R5 neurons. To access R5 cell bodies the flies were positioned in a horizontal holder and a small opening was cut into the dorsal part of the head capsule. The head capsule was bathed in external saline as previously described3. The osmolarity was adjusted to 280–283 mOsm and the saline was bubbled with O2/CO2 (95%/5%). Patch pipettes (6–8 MW) were filled with internal solution as previously described3. The pH of the internal solution was adjusted to 7.2 ± 0.1 and the osmolarity was adjusted to 265 ± 2 mOsm. For the optogenetic activation with CsChrimson flies were stimulated with 625 nm (1 Hz, 5 ms pulses) through the microscope objective. After break-in a small hyperpolarizing current (1–3 pA) was injected. The resting membrane potential was corrected for the liquid junction potential. All signals were digitized at 10 kHz and filtered at 5 kHz. The analysis was performed using MATLAB 2020a.
Immunostaining
Adult brains from 3- to 8-day-old female flies were dissected in ice cold phosphate-buffered saline (PBS) and fixed for 30 min in 4% paraformaldehyde in PBS at room temperature. After fixation brains were washed 3 times for 30 min in PBS + 0.3 Triton X-100 (0.3% PBS-T) and then moved to a 0.3% PBS-T + 10% normal goat serum (NGS) blocking solution for 2 h at room temperature. We incubated the brains in the following primary antibodies for 2 days at 4 °C in 0.3% PBS-T supplemented with 5% NGS: anti-GFP (1:1,000, Thermo Fisher Scientific), anti-HA (1:100, Sigma-Aldrich), anti-GABA (1:500, Sigma-Aldrich), anti-ChAT (1:100, DSHB deposited by P. Salvaterra), anti-VAChT (provided by T. Kitamoto51). After 2 days on primary antibody, brains were washed 4–6 times for 30 min in 0.3% PBS-T and then incubated in the following secondary antibodies in 0.3% PBS-T supplemented with 5% NGS: anti-chicken Alexa488 (1:400, Thermo Fisher Scientific), anti-rabbit Cy5 (1:400, Thermo Fisher Scientific), anti-mouse Cy3 (1:400, Jackson ImmunoResearch), anti-rat Alexa568 (1:400, Thermo Fisher Scientific). Afterwards, brains were washed 4 to 6 times for 30 min with 0.3% PBS-T, mounted in VectaShield (Vector Laboratories) and imaged on a Leica SP5 confocal microscope.
Network simulations
To investigate the network configurations and mechanisms generating slow-wave oscillations and networks coherence within and between R5 and helicon, we simulated network interactions using a spiking neuron model introduced by Izhikevich52 (Fig. 4 and Extended Data Fig. 7). For detailed description of the computational model, please see Supplementary information.
Connectome analysis
Neuronal types can be found in neuprint v1.2.1 (https://neuprint.janelia.org) under the same name used in the figures, except for ring neurons (ER5, ER4, etc. in the connectome) and helicon cells (ExR1). In the connectivity plots, input weight contribution of a neuron (or set of neurons) A from a neuron (or set of neurons) B refers to the number of synaptic connections that A receives from B, normalized to the total number of input connections of A. Output weight contribution of A to B refers to the number of output synaptic connections from A to B, normalized by the total number of output connections of A. The 2D morphological renderings of neurons were visualized using Python. For the analysis of neighbouring synapses in EPG neurons, we located individual R5-to-EPG and helicon-to-EPG input connections in every EPG. Then, we identified the closest input connection to each R5-to-EPG and helicon-to-EPG connection in the same neuron (excluding other connections from R5 and helicon cells, respectively and connections from cells not typed in the connectome). Distances between synaptic connections were calculated using geodesic (along the arbor) distance. For this analysis, EPG neurons included EPG and EPGt neuron types in neuprint.
Visualization, software and statistics
For all imaging recordings, compound activity was averaged across the whole structure. For R5, helicon and EPG the region of interest covered the whole ring, for the dFSB, the whole fan. Recordings were analysed using NOSA, a custom-made software specifically developed to analyse voltage and Ca2+ imaging recordings (see https://github.com/DavideR2020/NOSA). Relative fluorescence, background subtraction, power spectrum analysis, peak detection and cross-correlation analysis were performed with algorithms described in detail previously3,53. From NOSA, datasets were exported to Microsoft Excel for further analysis. For visualization of experimental data, we used CorelDRAW 2020. For a more intuitive display, all ArcLight and Varnam recordings are inverted, as depolarization leads to a decrease in fluorescence intensity while hyperpolarization leads to an increase. For power spectrum analysis, the unit of the PSD is indicated as PSD = [(ΔF/F)2/Hz] × 100. To quantify response amplitudes during optogenetic activation (Fig. 4h), we computed the difference of the average relative change in fluorescence intensity during and before optogenetic stimulation. All imaging data are presented as means with s.e.m.
For tracking recordings, single-fly position data were extracted from videos using EzTrack54 (https://github.com/denisecailab/ezTrack). Hemibrain connectome data (v.1.2.1)22 were accessed using neuprint-Python (https://github.com/connectome-neuprint/neuprint-python) and geodesic (along the arbor) distances between synaptic connections were calculated using navis (https://github.com/navis-org/navis). Manual annotation of behaviours was performed using BORIS (https://github.com/olivierfriard/BORIS)55. Behavioural and connectome data were analysed using custom-made Python scripts. In behavioural panels, box plots show median (line), quartiles (boxes) and range (whiskers) and locomotor activity curves indicate mean ± s.e.m. Behavioural and connectome data were visualized using Python.
R5 and helicon network simulations were performed, analysed and visualized using Python.
Statistical analysis was carried out in GraphPad Prism 8. The statistical tests performed in each panel are indicated in the figure legends. For unpaired comparisons, we used Mann–Whitney test when comparing two groups and Kruskal–Wallis test with Dunn’s post hoc analysis for multiple comparisons. For paired comparisons, we used a paired t-test and Wilcoxon matched pairs signed-rank test when comparing two groups and Friedman test with Dunn’s post hoc analysis for multiple comparisons. Comparisons to 0 were performed using Wilcoxon signed-ranked tests and percentages were compared using Binomial test.
In figure captions, n refers to the number of flies tested, unless specified elsewise.
Sample sizes were chosen on the basis of previous studies3. We did not explicitly randomize or blind experimental groups.
Statistics and reproducibility
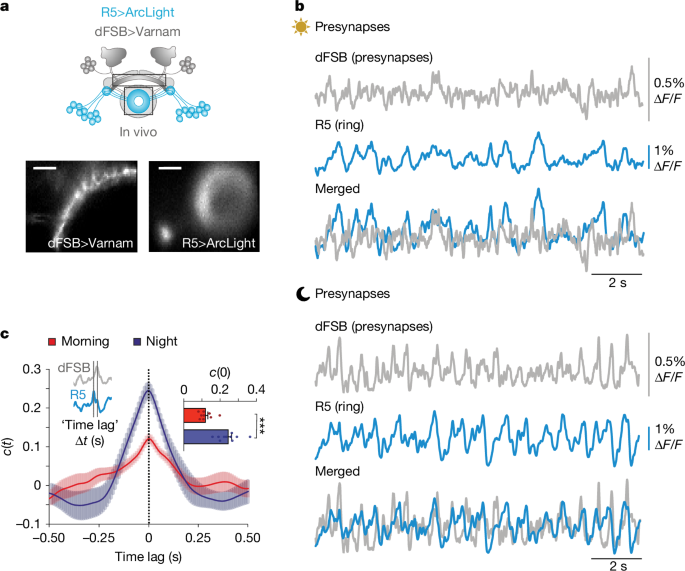
All wide-field images shown in Figs. 1a, 3b and 5c are representative images and have been collected for each performed recording. Images shown in Extended Data Fig. 6b,c are representative of four independent experiments. The experiment in Extended Data Fig. 6e–g is an independent confirmation of the experiments performed in Extended Data Fig. 6b,c and was performed once.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.