Cell strains
The E. coli K-12 MG1655 strain was grown in Luria–Bertani medium at 37 °C with shaking at 200 rpm until reaching an OD600 of approximately 0.6. For heat shock treatment, cells were incubated in a water bath at 44 °C for 10 min. For transcription inhibition, cells were treated with 25 or 750 µg ml−1 Rif for 40 min at 37 °C and 200 rpm. To induce DNA damage, cells were exposed to 70 µg ml−1 bleomycin for 10 min at 37 °C and 200 rpm. For DNA gyrase inhibition, cells were treated with 0.025 or 0.25 µg ml−1 ciprofloxacin or 25 or 250 µg ml−1 novobiocin for 15 min at 37 °C and 200 rpm. For netropsin treatment, cells were grown to an OD600 of approximately 0.4, followed by the addition of netropsin to a final concentration of 20 or 200 µg ml−1 and incubation for 30 min at 37 °C and 200 rpm.
Construction of deletion mutants
The E. coli Δhns-knockout strain was generated using a modified λ-Red phage-assisted recombineering approach56. The phage λ att-L and att-R site-flanked chloramphenicol (Cm) resistance cat gene57 was amplified by PCR using primers with 36-nucleotide extensions homologous to the upstream and downstream chromosomal sequences of hns. The purified PCR product was electroporated into E. coli MG1655 cells carrying the pKD46 plasmid, which encodes the λ-Red recombination genes bet, exo and gam. To induce λ-Red genes, 50 mM l-arabinose was added to SOB cultures with ampicillin (Ap), and cells were grown to an OD600 of approximately 0.5 at 30 °C. Electrocompetent cells were prepared using the glycerol–mannitol density step centrifugation method58. Recombinant hns::Cmr cells were selected on LB agar plates containing Cm and verified by PCR with specific primers. The pKD46 plasmid was cured by growth at 37 °C, and CmrAps clones were selected. To eliminate the cat gene flanked by λ att-L and att-R sites, mutants were transformed with the pInt/Xis plasmid, which encodes the λ Int/Xis-dependent recombination system57,59. Ap-resistant transformants were selected at 30 °C and verified by PCR for the loss of the cat gene and the deletion of the hns gene. The clones were colony purified at 37 °C and tested for Ap and Cm sensitivity to confirm the loss of both the helper plasmid and the cat gene.
E. coli MG1655 fis::Kmr and stpA::Kmr deletion mutants were generated by transducing the corresponding alleles from the Keio collection60 into MG1655 using phage P1vir, followed by selection for kanamycin resistance. To eliminate FRT-flanked Kmr cassette from the fis::Kmr strain, the pCP20 plasmid56 was used. Ampicillin-resistant transformants were selected at 30 °C, then purified without selection at 37 °C to cure the helper plasmid. Successful removal of all antibiotic resistance markers was confirmed.
The stpA::Kmr region was moved into the Δhns strain by P1 transduction, generating the double knockout strain through selection for kanamycin.
The muk(FEB)::Cmr strain, in which all three genes of the mukF–mukE–mukB operon were knocked out, was constructed using the same method as for the hns deletion mutant. A relevant pair of primers with 36-nucleotide extensions homologous to the start of mukF and the end of mukB was used. Owing to the temperature-sensitive phenotype resulting from muk(FEB) inactivation, all incubations were performed at 20–22 °C. After selection for Cmr, the pKD46 was cured from the strain by overnight growth in LB medium without antibiotics at 20 °C, followed by plating on LB agar with Cm. Colonies were screened for ampicillin sensitivity and verified by PCR to confirm insertion of the Cmr cassette in place of the muk(FEB) genes. The resulting strain forms colonies only at 22 °C and fails to grow at 30 °C or 37 °C.
Growth curves
Individual colonies of E. coli strains were cultured in LB medium to the stationary phase and then diluted with fresh LB medium to an OD600 of approximately 0.08. Cultures were grown at 37 °C until reaching an OD600 of approximately 0.4. A 150 µl aliquot of each culture, further diluted to an OD600 of approximately 0.01, was transferred into honeycomb wells and incubated at 37 °C with vigorous shaking using the Bioscreen C automated growth analyzer. OD600 values were recorded automatically every 10 min, and growth curves were plotted based on the collected data.
Micro-C
Micro-C was adapted from ref. 11. E. coli cell cultures (2–3 ml) were transferred to PBS-diluted formaldehyde (28906, Thermo Fisher) to achieve a final formaldehyde concentration of 3% and a total volume of 13.5 ml. Fixation was performed for 30 min at room temperature with gentle rotation, followed by quenching with 375 mM Tris (pH 7.5).
Previous studies have shown that H-NS bridging is temperature dependent, with maximum bridging observed below 30 °C (ref. 61). To determine whether the detection of H-NS-dependent features in Micro-C contact maps depends on cultivation or fixation temperature, we compared contact maps for cells cultivated and fixed at room temperature, cells cultivated and fixed at 37 °C, and cells cultivated at 37 °C but fixed at room temperature following the standard protocol. No differences in the intensity of CHINs were observed between the three conditions (Extended Data Fig. 9b–d).
Following formaldehyde crosslinking, cells were centrifuged at 3,220g for 20 min at 4 °C, resuspended in 1 ml of cold PBS and centrifuged again at 16,100g for 5 min at 4 °C. The cell pellet was resuspended in 205 µl PBS and mixed with an equal volume of PBS-diluted disuccinimidyl glutarate (DSG) (20593, Thermo Fisher; used as 300 mM stock in DMSO, freshly made) to a final DSG concentration of 2 mM. Fixation was carried out for 45 min at room temperature with rotation, followed by quenching with 375 mM Tris (pH 7.5).
To assess whether DSG crosslinking is effective in E. coli, we compared it with disuccinimidyl suberate (DSS), a cell-permeable crosslinker commonly used in E. coli studies62,63. Both crosslinkers produced a similar number of protein–protein crosslinks in E. coli cells (Extended Data Fig. 9e). In addition, the inclusion of DSG was essential for stabilizing Micro-C material, as MNase treatment of cells fixed with formaldehyde alone resulted in the solubilization of all DNA fragments (Extended Data Fig. 9f).
After DSG crosslinking, cells were centrifuged at 16,100g for 5 min at 4 °C, washed with 1 ml cold PBS and centrifuged again. Cells were resuspended in 250 µl of TE buffer (10 mM Tris pH 8.0 and 1 mM EDTA) supplemented with 1× protease inhibitors (Bimake). Lysozyme (L6876, Sigma) was added to a final concentration of 4 µg ml−1, and the mixture was incubated at 37 °C with shaking at 1,000 rpm for 30 min. Cells were then pelleted at 16,100g for 5 min at room temperature and resuspended in MNase buffer (10 mM Tris pH 7.5 and 1 mM CaCl2). MNase (EN0181, Thermo Fisher; used as 2 or 5 U µl−1 stock in 20 mM Tris pH 7.5, 50 mM NaCl and 50% glycerol) was added and samples were incubated for 25 min at 37 °C with shaking at 1,000 rpm. The reaction volume ranged from 475 µl to 1,160 µl, and MNase amounts varied from 3 U to 12 U, with conditions optimized individually for each sample to obtain DNA fragments ranging from approximately 75 to 1,000 bp (Extended Data Fig. 9g).
Samples showing excessive DNA degradation, characterized by reduced DNA yield, were typically discarded (for example, sample 4 rep1 in Extended Data Fig. 9h). However, even heavily overdigested samples retained characteristic Micro-C contact patterns (Extended Data Fig. 9i), demonstrating the robustness of the procedure despite varying levels of MNase digestion.
DNA digestion was terminated by adding EGTA to 5 mM followed by centrifugation for 5 min at 16,100g at room temperature. The pellet was resuspended in 220 µl 1× PNK buffer (NEB), and 20 µl was set aside and subjected to DNA purification for MNase digestion control (Fig. 1a). The remaining sample was centrifuged for 5 min at 16,100g at room temperature and resuspended in 145 µl 1.03× PNK buffer (NEB) followed by adding 5 µl PNK (10 U µl−1, NEB) to dephosphorylate DNA 3′ ends. The mixture was incubated for 30 min at 37 °C with shaking at 900 rpm and centrifuged as above. The sample was resuspended in 90 µl solution composed of 10 µl 10× NEB buffer 2.1, 2 µl 100 mM ATP, 5 µl 100 mM dithiothreitol and 73 µl water. The mixture was supplemented with 5 µl PNK (10 U µl−1, NEB) and incubated for 15 min at 37 °C with shaking at 900 rpm to phosphorylate DNA 5′ ends. The mixture was then supplemented with 5 µl Klenow (5 U µl−1, NEB) and incubated for 15 min at 37 °C with shaking at 900 rpm to generate 3′ recessed DNA ends in the absence of dNTPs. The 3′ recessed DNA ends were labelled with biotin by adding 50 µl solution composed of 5 µl 10× T4 DNA ligase buffer (NEB), 10 µl 1 mM biotin–dATP, 10 µl 1 mM biotin–dCTP, 1 µl 10 mM dGTP, 1 µl 10 mM dTTP, 0.25 µl BSA (10 mg ml−1, NEB) and 22.75 µl water. The mixture was incubated for 45 min at 25 °C with shaking at 900 rpm and centrifuged as above.
The pellet was resuspended in 95 µl 1.05× T4 DNA ligase buffer (NEB) followed by adding 5 µl PNK (10 U µl−1, NEB) and incubating for 1 h at 37 °C with shaking at 900 rpm to ensure complete phosphorylation of DNA 5′ ends. The sample was pelleted as above and resuspended in 478 µl 1.02× T4 DNA ligase buffer (Thermo Fisher) followed by adding 12.5 µl T4 DNA ligase (5 Weiss U µl−1, Thermo Fisher). DNA proximity ligation was carried out overnight at 22 °C with rotation, and the mixture was centrifuged as above. The pellet was resuspended in 148.5 µl 1.01× NEB buffer 1 followed by adding 1.5 µl exonuclease III (100 U µl−1, NEB) and incubating for 15 min at 37 °C with shaking at 900 rpm to remove biotin from non-ligated ends.
To reverse crosslinks, the sample was supplemented with 125 µl solution composed of 15 µl 10× NEB buffer 2, 4.5 µl 5 M NaCl, 30 µl 10% SDS and 75.5 µl water followed by adding 25 µl proteinase K (20 mg ml−1, Ambion) and incubating for 1 h at 55 °C and then for 7 h at 65 °C. The sample was extracted with an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1), pH 8.0, followed by centrifugation for 5 min at 12,000g and room temperature. The water phase was supplemented with 1/10 volume 3 M NaAc, 3 µl glycogen (20 mg ml−1, Thermo Fisher) and 2.5 volumes 96% ethanol. The sample was incubated for 30 min at room temperature and centrifuged for 30 min at 21,000g and 4 °C. The pellet was washed with 1 ml cold 70% ethanol and centrifuged for 10 min at 21,000g and 4 °C. The pellet was air dried and diluted in 45 µl 10 mM Tris pH 8.0. RNase A (10 mg ml−1, Thermo Fisher) was added to 40 µg ml−1 and the sample was incubated for 30 min at 37 °C. A 5 µl aliquot was set aside for ligation control (Fig. 1a). The remaining sample was further purified with 2 volumes of AMPure XP beads (Beckman Coulter) and finally eluted into 40 μl 10 mM Tris pH 8.0 followed by measuring DNA concentration with the Qubit dsDNA broad range kit. A typical DNA yield was 1–2 μg.
The sample was brought to 250 µl by water followed by adding 250 µl 2× sonication buffer (100 mM Tris pH 8.0, 20 mM EDTA and 0.2% SDS). The sample was sonicated on ice with five 30-s pulses followed by 3-min rest periods using a VirTis VirSonic 100 sonicator at high power (setting 15). The sample was passed through Amicon 30 K Ultra-0.5-ml centrifugal filters (Millipore) by centrifugation for 5 min at 16,100g and 4 °C. Then, 450 μl 10 mM Tris pH 8.0 was added into the filter and centrifugation was repeated. Another 450 μl 10 mM Tris pH 8.0 was added into the filter and centrifugation was repeated. The concentrated sample was removed from the filter, brought to 100 µl by 10 mM Tris pH 8.0 and subjected to biotin pull-down.
For this process, 10 μl Dynabeads MyOne Streptavidin C1 beads (10 mg ml−1, Thermo Fisher) was washed twice with 400 μl Tween washing buffer (TWB; 5 mM Tris pH 7.5, 0.5 mM EDTA, 1 M NaCl and 0.05% Tween 20) by repeating the resuspension–magnet separation. Streptavidin beads were resuspended in 100 μl 2× binding buffer (10 mM Tris pH 7.5, 1 mM EDTA and 2 M NaCl) and mixed with sonicated DNA followed by incubation for 15 min at room temperature. Streptavidin beads with tethered DNA fragments were washed twice with 600 μl TWB, once with 100 μl 1× NEB buffer 2 and once with 100 μl 1× T4 DNA ligase buffer (NEB), and resuspended in 75 μl end repair solution containing 1× T4 DNA ligase buffer (NEB), 0.25 mM each dNTP, 5 μl PNK (10 U μl−1, NEB), 1 μl Klenow (5 U μl−1, NEB) and 4 μl T4 DNA polymerase (3 U μl−1, NEB). The mixture was incubated for 30 min at 22 °C. Streptavidin beads were washed twice with 600 μl TWB and twice with 100 μl 1× NEB buffer 2, and resuspended in 95 μl 1.05× NEB buffer 2 supplemented with 0.21 mM dATP. The mixture was supplemented with 5 μl Klenow exo-minus (5 U µl−1, NEB) and A-tailing was carried out for 40 min at 37 °C. Streptavidin beads were washed twice with 600 μl TWB, once with 100 μl 1× NEB buffer 2 and once with 100 μl 1× T4 DNA ligase buffer (Thermo Fisher), and resuspended in 46.5 μl 1.08× T4 DNA ligase buffer (Thermo Fisher). The mixture was supplemented with 2.5 μl Illumina TruSeq single index adapter and 1 μl T4 DNA ligase (5 Weiss U µl−1, Thermo Fisher) and incubated for 2.5 h at 22 °C with occasional mixing. After ligation, streptavidin beads were washed twice with 600 μl TWB, once with 100 μl 1× NEB buffer 2 and once with 100 μl 10 mM Tris pH 8.0, and resuspended in 16 μl water.
DNA was amplified in 50 μl PCR containing 1× KAPA HiFi Fidelity Buffer, 0.3 mM each dNTP, 0.5 μM Illumina forward primer, 0.5 μM Illumina reverse primer, 1 U KAPA HiFi DNA polymerase and 4 μl streptavidin beads from the above step. PCR was performed as follows: 95 °C for 5 min, [98 °C for 20 s, 65 °C for 15 s and 72 °C for 20 s] × 8–10 cycles, and 72 °C for 3 min. For ligase minus control, 12 PCR cycles were used. PCR products of two reactions were pooled and purified twice with 1.2 volumes of AMPure XP beads. Purified PCR products were paired-end sequenced on the Illumina NovaSeq or BGI DNBSEQ-T7 platforms.
Micro-C read processing
Sequencing reads were processed using distiller-nf (v0.3.4). Mapping was performed using BWA with default pipeline parameters to the reference genome NC_000913.3. Statistics of read filtering and mapping are presented in Supplementary Table 2.
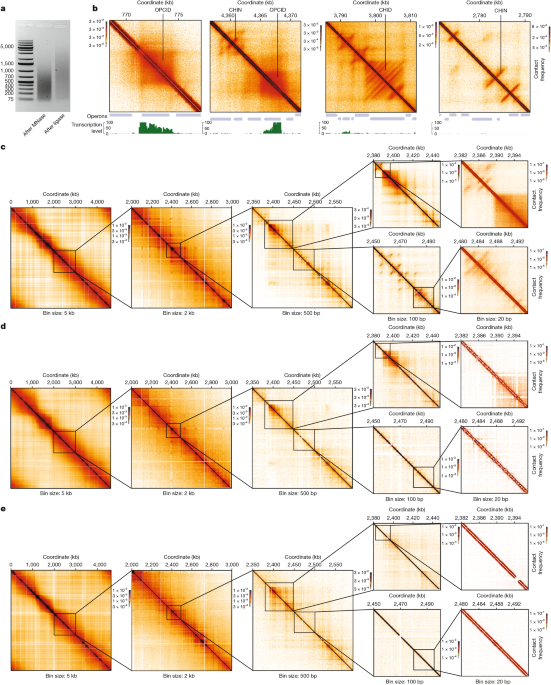
The generation of a large number of reads (approximately 1.5 billion for main samples) and the relatively small size of the E. coli genome enabled us to construct DNA–DNA contact maps with a resolution of up to 10 bp. At this resolution, CHINs remained detectable in contact maps; however, further increases in resolution resulted in the structures becoming less discernible (Extended Data Fig. 1a).
The sample correlation was computed using HiCRep64 with a smoothing parameter h = 30 and resolution of 100 bp.
Scalings were calculated using the expected cis module from cooltools (v0.7.0)65, using the following parameters: smooth = True, aggregate_smoothed = True. Contacts at distances less than 100 bp were excluded. The resulting plot was generated using double logarithmic coordinates.
Operons
We use operons from RegulonDB66 (Supplementary Table 3). The information on sigma factors was extracted from the annotated TSS dataset from RegulonDB. The level of transcription activity was inferred from the Red-C data (see below).
In the piles-up analysis (Figs. 2b and 3b and Extended Data Fig. 4), we used the top 10% active operons and operons with zero Red-C signal (inactive operons). In the average contact map analysis presented in Fig. 2a, operons were divided into four groups by the Red-C signal (bottom 50%, 50–75%, 75–90% and top 10%) followed by selecting only operons with the length higher than the median length of all operons (1,343 bp) to reduce the contribution of the Micro-C signal near the diagonal. In the average contact map analysis of σ32 operons (Fig. 2e), we also used operons longer than 1,343 bp (26 out of 44). In the analysis of promoter–terminator contacts presented in Fig. 2g, operons were grouped by transcription activity, determined by the Red-C signal normalized to feature length.
Annotation of CHINs, CHIDs and OPCIDs
Manual annotation of CHINs, CHIDs and OPCIDs was performed on the contact map for WT E. coli cells under normal growth conditions (37 °C). The contact map was examined using HiGlass browser in ‘blind’ mode, with no operons or other tracks displayed to avoid bias during the search. In annotating CHINs and CHIDs, we aimed to identify as many instances as possible, whereas only a representative fraction of OPCIDs was annotated. We also focused on identifying CHIN contacts by searching for elongated spots located away from the diagonal. The coordinates of the annotated OPCIDs, CHINs, CHIDs and CHIN contacts can be found in Supplementary Tables 4–7.
To validate the accuracy of the manual annotation of CHINs, we also used the Chromosight program67 for annotation (Supplementary Table 8). We found a good correlation between the positions of the manually annotated and Chromosight-annotated CHINs (Extended Data Fig. 1e,f). The discrepancies between the two sets may be attributed to false positives and false negatives present in the Chromosight dataset (Extended Data Fig. 1e). Of note, CHINs identified by Chromosight also exhibited the characteristic bimodal distribution of H-NS around them (Extended Data Fig. 1g).
Average contact map
To create an average contact map, we utilized coolpuppy (v1.1.0)68 with the following parameters: local = True, rescale = True, rescale_size = 401 and rescale_flank = 1. For the observed-over-expected maps, contact frequencies were normalized by dividing them by ‘expected’ values, which were calculated using the expected_cis function from cooltools (v0.5.4) with ignore_diags = 2. The plotpup module from coolpuppy was used to generate figures.
The average contact maps were generated from Micro-C contact maps with a resolution of 10 bp. Using lower-resolution Micro-C maps for plotting the average map leads to more blurred features and thicker diagonal (Extended Data Fig. 1c,d).
TSS–TES contacts calculation
To calculate the observed number of TSS–TES contacts for each operon, we summed the ICE-balanced contacts between the 40 bp region (4 × 10 bins): one centred at the TSS and the other at the TES. To estimate the expected number of contacts for an operon, we selected 10 random genomic intervals of the same length as the operon. We then calculated the contact counts between the ends of these intervals using the same method and averaged the values to obtain the expected contact count.
As a control, we also calculated the number of contacts between more internal regions of the operon (intra-operon contacts). Specifically, we selected a region located 20% of the operon length downstream of the TSS and another region 20% of the operon length upstream of the TES (Fig. 2g).
The observed–expected frequencies of TSS–TES and intra-operon contacts are provided in Supplementary Table 3.
Piles-up
The average ChIP–seq signal around features were plotted using the custom function plot_around_loop with some imports from the pybbi package (v0.4.0). The following length of flanking regions was used: 1,000 bp (Extended Data Fig. 1g) and 500 bp (all other piles-up).
We used the following ChIP–seq datasets: GSE157512 for H-NS, H-NS under Rif, StpA and RpoB; GSE182473 for TopoI; GSE182079 for GyrA and GyrB; E-MTAB-332 (Array Express) for Fis; GSE181767 for MukB, HupA and HupB; and GSE152880 for GapR.
ChIP–seq for TopoI was initially aligned to the W3110Mu assembly, so the liftOver chain file was generated using pyoverchain to transfer the signal to the NC_000913.3 assembly.
Shuffle test
To assess whether a genomic feature (\(A\)) non-randomly colocalizes with or avoids another feature (\(B\)), a shuffle (permutation) test was conducted. For a summary of the procedure:
-
(1)
The intersection between features \(A\) and \(B\) (\(A\cap B\)) was calculated using bedtools intersect with a fraction overlap threshold of 0.1 (option f = 0.1).
-
(2)
To perform shuffling, bedtools shuffle was utilized, with the option chrom=True to maintain the chromosome structure while randomizing the locations of the features.
-
(3)
For each shuffle, the intersection between the shuffled feature \(A\) and feature \(B\) (\(A\cap B\)) was calculated.
-
(4)
The number of shuffle iterations was set to 1,000 (\({N}_{s}\) = 1,000).
-
(5)
The P value was computed as the minimum of the ratios \({N}_{{A}_{s}\cap B > A\cap B}\)/\({N}_{s}\) and \({N}_{{A}_{s}\cap B < A\cap B}\)/\({N}_{s}\), where \({N}_{{A}_{s}\cap B > A\cap B}\)/\({N}_{s}\) represents the number of shuffles where the intersection between shuffled \(A\) and \(B\) is greater than the number of observed intersection \(A\cap B\), and \({N}_{{A}_{s}\cap B < A\cap B}\)/\({N}_{s}\) represents the number of shuffles where the intersection is smaller than the observed intersection.
To assess the colocalization of H-NS peaks with hairpins, we used H-NS peaks from the GSE157512 dataset. To assess the colocalization of HTGs with hairpins, we retrieved HTGs from the database HGT-DB47 (https://usuaris.tinet.cat/debb/HGT/ecoli.d/HGTList.html).
Motifs
The motif analysis was performed using HOMER (v4.11.1). The identified motifs were compared with the prodoric_2021.9.meme database using tomtom (v5.5.5) from the MEME suite.
RNA sequencing
Total RNA was isolated from 0.5 ml cell culture using the Qiagen RNease Mini RNA Isolation Kit, following the manufacturer’s protocol. rRNA was removed from 200 ng of the starting material using the NEBNext rRNA Depletion Kit (bacteria), and libraries were prepared using the NEBNext Ultra II Directional RNA-Seq library Preparation Kit. Libraries were sequenced on an Illumina Nextseq 2000 instrument in paired-end mode (2 × 61 bp) to a depth of approximately 20 million read pairs per sample. Data processing was conducted using the nf-core/rnaseq (v3.16.0) workflow (https://doi.org/10.5281/zenodo.1400710), part of the nf-core collection69, utilizing reproducible software environments from Bioconda70 and Biocontainers71. Differential gene expression analysis and data visualization were performed with the DESeq2 package72.
RT–qPCR
Cell cultures were mixed with ice-cold ethanol–phenol stop solution to inactivate cellular RNases, pelleted at 4 °C, washed with cold saline and stored at −80 °C. Total RNA was extracted using the hot phenol method73, and RNA quality was assessed by agarose gel electrophoresis and the Qubit RNA IQ assay. RNA quantitation was performed with the Qubit RNA BR Assay Kit. RNA samples were treated with ezDNase (Invitrogen) to remove contaminating genomic DNA. Complementary DNA synthesis was performed using 0.25 µg of total RNA, MMLV reverse transcriptase (Evrogen) and specific oligonucleotide primers according to the manufacturer’s instructions. RT–qPCR was performed using qPCRmix-HS SYBR (Evrogen) on a QuantStudio 5 Real-Time qPCR system (Applied Biosystems). rpoC (RNA polymerase β′-subunit) and rrn (pre-rRNA) served as housekeeping genes. All samples were normalized to the 3′ end of 16S rRNA, and fold changes in expression levels were calculated using the Pfaffl method74.
Red-C
Red-C was performed as described38 with minor modifications relating to cell fixation and lysis conditions. Cell culture (5 ml) was transferred to 35 ml PBS-diluted formaldehyde (F8775, Sigma-Aldrich) to obtain a final formaldehyde concentration of 3%. Fixation was allowed to proceed for 30 min at room temperature with rotation followed by quenching with 375 mM glycine. Cells were centrifuged for 10 min at 3,220g and 4 °C, washed with 10 ml cold PBS and centrifuged again.
Cells were resuspended in 500 µl TE (10 mM Tris pH 8.0 and 1 mM EDTA) supplemented with 1× protease inhibitors (Bimake) and 100 U SUPERase.In RNase inhibitor (Invitrogen) followed by adding lysozyme (L6876, Sigma) to 4 µg ml−1. The mixture was incubated for 30 min at 37 °C with shaking at 1,000 rpm followed by adding 25 µl 10% SDS and incubating for 15 min at 37 °C with shaking at 1,000 rpm. The sample was supplemented with 500 µl TE and 87 µl 20% Triton X-100 followed by incubation for 15 min at 37 °C with shaking at 1,000 rpm. After adding 370 µl 4× NEB buffer 4, the sample was pelleted for 20 min at 16,100g and room temperature and resuspended in 500 µl 1× NEB buffer 4. DNA was digested with 20 µl NlaIII (10 U µl−1, NEB) for 2 h at 37 °C with shaking at 1,200 rpm. Downstream steps of the Red-C procedure including RNA-to-DNA bridge ligation, reverse transcription, library construction and sequencing were done as previously described38. The raw Red-C reads were processed using the RedClib computational pipeline (https://github.com/agalitsyna/RedClib) as previously described38.
Estimation of transcription level from Red-C data
The Red-C data on RNA–DNA contacts obtained in this work were used to estimate the level of transcription in operons and other genomic features (CHINs, CHIDs and OPCIDs), as previously suggested38. As a measure of transcriptional activity of a feature, we calculated the number of contacts that RNA transcribed from this feature establishes near the feature (the distance between mapping coordinates of RNA and DNA reads was required to be no more than 10 kb). We reasoned that RNA captured near its parental locus may better convey the transcriptional activity of this locus than, for example, the total RNA output as determined by RNA sequencing. Of note, a good correlation has been shown between the level of a transcript in the RNA sequencing data and the number of contacts it showed in Red-C data38. The Red-C signal for operons, OPCIDs, CHINs and CHIDs is presented in Supplementary Tables 3–6, respectively. We also generated transcription activity profiles by calculating for each genomic bin how many contacts the RNA transcribed from this bin establishes near the bin (Extended Data Fig. 10).
Hi-C
Hi-C was performed as previously described75 with minor modifications. Cell fixation, lysis and restriction enzyme digestion were performed as described above for Red-C with the only difference that 10 µl HpaII (50 U µl−1, NEB) was used instead of NlaIII and the digestion was carried out for 3 h instead of 2 h.
After HpaII digestion, the sample was pelleted for 10 min at 21,000g and 10 °C and resuspended in 250 µl 1× NEB buffer 2 followed by adding 42 µl fill-in solution composed of 15 µl 1 mM biotin–dCTP, 1.5 µl 10 mM dAGTTP (mix of dATP, dGTP and dTTP at 10 mM each) and 25.5 µl water. The mixture was supplemented with 8 μl Klenow (5 U μl−1, NEB) and incubated for 2 h at 25 °C with shaking at 900 rpm. The sample was pelleted as above and resuspended in 300 µl 1× T4 DNA ligase buffer (Thermo Fisher) followed by adding 12.5 µl T4 DNA ligase (5 Weiss U µl−1, Thermo Fisher). DNA proximity ligation was carried out overnight at 20 °C with shaking at 1,000 rpm. The sample was pelleted as above and resuspended in 375 µl proteinase K buffer composed of 4 µl 1 M Tris pH 8.0, 8 µl 5 M NaCl, 1.6 µl 0.5 M EDTA, 40 µl 10% SDS and 321 µl water followed by adding 25 µl proteinase K (20 mg ml−1, Ambion) and incubating for 8 h at 65 °C. Downstream steps of the Hi-C procedure including DNA purification, sonication, biotin pulldown, library construction, sequencing and read processing were done as described above for Micro-C.
In vivo crosslinking and mass spectrometry
In vivo crosslinking and mass spectrometry-assisted identification of covalent crosslinks formed by DSG were performed as previously described62, with minimal modifications. In brief, E. coli MG1655 rpoC::His10 cells were grown in 0.5× terrific broth (Difco) at 37 °C with shaking at 250 rpm to an OD600 of approximately 0.5. The culture was then supplemented with a 300 mM stock solution of DSG (CF Plus) in DMSO, to a final concentration of 2 mM. After addition of DSG, the culture was incubated with shaking at 37 °C for 30 min, followed by quenching with 20 mM Tris-HCl pH 7.5. Cells were harvested by centrifugation and processed as previously described62. DSG-crosslinked RNA polymerase was purified by a combination of Ni-affinity and size-exclusion chromatography. Crosslinked peptides were identified from mass spectrometry raw data using pLink2 software62. For comparative analysis of crosslinking efficiency, data obtained from DSS-crosslinked samples prepared under identical conditions were analysed in parallel62.
H-NS cloning and purification
The open reading frame of the E. coli H-NS protein was cloned into the pTYB-1 vector (NEB) and transformed into Bl21 (DE3) cells. A single colony was used to inoculated 5 ml of LB medium supplemented with 100 mg ml−1 carbenicillin, and the culture was incubated overnight at 37 °C. The following day, 0.5 ml LB medium of overnight culture was used to inoculate two 0.5 l LB cultures in 2-l flasks. Each flask was supplemented with 25 ml of 20× NPS solution (0.5 M (NH4)2SO4, 1 M KH2PO4, 1 M Na2HPO4; final pH approximately 6.75), 10 ml of 50× 5052 autoinduction solution (25% glycerol, 2.5% glucose and 10% lactose) and 0.5 ml of 1 M MgSO4. Cultures were grown overnight at 30 °C in the presence of 100 µg ml−1 carbenicillin following the autoinduction protocol76. Cells were collected by centrifugation at 5,000g and the pellet was resuspended in 75 ml of chitin–intein column (CIC) buffer (20 mM Tris-HCl pH 8.0, 500 mM NaCl and 1 mM EDTA) containing two protease inhibitor cocktail tablets (Roche). Lysozyme was added to a final concentration of 0.5 mg ml−1 and the suspension was incubated at 37 °C for 30 min with gentle stirring. Sodium deoxycholate was then added to 0.05% and stirring continued for another 15 min. The suspension was sonicated on ice, and the lysate was clarified by centrifugation at 20,000g for 30 min and 4 °C. The cleared lysate was applied by gravity flow to a chitin–agarose column (approximately 5 ml dry bed, NEB) pre-equilibrated in CIC buffer at room temperature. After washing with 60 ml CIC, the column was incubated with 15 ml of CIC buffer supplemented with 50 mM dithiothreitol to induce on-column intein-mediated cleavage. The column was sealed and left overnight at room temperature. The following day, the eluted protein was collected with 20 ml CIC buffer. The eluate was concentrated using an Amicon centrifugal filter unit (15 ml, 3 kDa MWCO) at 5,000g and 4 °C to a final concentration of approximately 0.7–1 ml. The protein was diluted 1:1 with glycerol and stored at −80 °C.
Transmission electron microscopy
The control DNA template (plasmid without CHIN) was pVS10 (ref. 77) containing the E. coli genes rpoA, rpoB and rpoC. To generate a CHIN-containing plasmid, the rpo gene region of pVS10 was replaced via Gibson Assembly (NEB) with a genomic fragment encompassing yqeG, yqeH, yqeL, yqeJ, yqeK, ygeG, ygeH and ygeL (E. coli coordinates 2,985,045–2,996,445; Extended Data Fig. 5e,f). Plasmids were purified using the Qiagen Midiprep kit. To relax supercoiling, plasmids were nicked before imaging. For the control plasmid, nickase Nb.BbvCI (NEB) was used; for the CHIN-containing plasmid, Nt.BspQI (NEB) was used. Nicked DNA was purified by phenol–chloroform extraction and ethanol precipitation (no carrier).
H-NS–DNA complexes were formed following a modified version of a previously described protocol78. In brief, 100 ng of plasmid DNA was incubated with 420 ng of purified H-NS in 10 µl of binding buffer (40 mM HEPES pH 8.0, 10 mM MgCl2 and 60 mM KCl) for 30 min at 30 °C. The reaction mixture was then diluted 20-fold with a deposition buffer to a final concentration of 5 mM HEPES pH 8.0, 5.5 mM MgCl2 and 3 mM KCl and applied to glow-discharged carbon-coated copper transmission electron microscopy grid (PELCO, 01844-F, Ted Pella Inc.). In some experiments, the dilution step was omitted to increase the density of visualized molecules (Extended Data Fig. 5h,i). Plasmid without H-NS was prepared and deposited under identical conditions. Grids were negative stained with 1% (w/v) uranyl acetate for 5 min, air dried and imaged using a FEI Talos 120 C transmission electron microscope equipped with a Gatan OneView 4k × 4k camera.
PLA
Construction of the dCas9 expression plasmid was based on the pdCas9-bacteria plasmid79, modified by Gibson Assembly to append a carboxy-terminal 6×histidine (6×His) tag. Dual small guide RNA (sgRNA) expression cassettes were constructed by replacing the single sgRNA module of the pgRNA-bacteria plasmid79 with synthetic DNA (Twist Bioscience) encoding extra-long sgRNA arrays (ELSA) as previously described80.
Each dual cassette included the following elements: SHP050 promoter – sequence-specific gRNA1 spacer – ID46 Handle – ECK120010868 terminator – SHP038 promoter – sequence-specific gRNA2 spacer – ID26 Handle – ECK120017009 terminator. Three dual cassettes were assembled to target combinations A–C, B–C or D–C (Extended Data Fig. 5b). In all constructs, gRNA2 targeted position C, whereas gRNA1 targeted positions A, B or D. Plasmids encoding dCas9-6×His and the dual ELSA arrays were sequentially transformed into E. coli strain MG1655 to generate the strains used in PLA experiments. Full sequences of all cassettes are listed in Supplementary Table 9.
PLA was performed as previously described81,82,83. Strains carrying both plasmids were grown in LB medium at 37 °C to an OD600 of approximately 0.3, then induced with 0.1 μg ml−1 anhydrotetracycline (aTc) to express dCas9-6×His. After 30 min of incubation, cells were harvested by centrifugation and washed three times with PBS. Pellets were resuspended in PBS and immobilized on cleaned poly-l-lysine-coated coverslips for 30 min. Cells were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature. After fixation, cells were lysed with a buffer consisting of 25 mM Tris pH 8.0, 10 mM EDTA, 0.5% Triton X-100 and 0.1 mg ml−1 lysozyme, and incubated for 30 min with gentle agitation. Samples were washed once with PBS and then subjected to the Duolink In Situ PLA protocol (Millipore Sigma), using mouse anti-6×His antibodies (66005-1-Ig, Proteintech) and rabbit anti-6×His antibodies (ab9108, Abcam), each at 1:300 dilution. Duolink PLA probes and detection reagents (Millipore Sigma) were used per the manufacturer’s instructions.
Fluorescence imaging was performed on a Zeiss LSM700 confocal microscope. The number of PLA foci per cell was quantified in an unbiased manner using ImageJ (Extended Data Fig. 5d).
To confirm DNA colocalization of PLA foci, coverslips prepared for confocal imaging were repurposed for super-resolution imaging on a custom-built optical system based on an ASI-RAMM inverted microscope, equipped for multilaser excitation81. The lasers were adjusted to a highly inclined and laminated optical sheet mode. Each colour was filtered using single-bandpass filters (FF01-676/37 and FF01-607/36 for AF647, Semrock), and filters were switched using a motorized filter wheel (FW-1000, ASI) for sequential illumination. Photons were collected with a Teledyne Photometrics Prime 95B sCMOS camera at 33 Hz (30 ms per frame) for a minimum of 1,000 frames. Images were rendered from localization coordinates on a 10-nm pixel canvas and displayed after Gaussian blurring (σ = 10 nm; Extended Data Fig. 5c).
Data reproducibility
All experiments were performed with a minimum of two biological replicates, except for the Micro-C experiments without added DNA ligase and those with varying cultivation or fixation temperatures, which were performed as single repeats. In addition, the Micro-C experiments with the antibiotics ciprofloxacin, novobiocin and netropsin were performed at two concentrations, with each concentration run as a single repeat.
The Micro-C contact maps showed strong concordance between replicates (Extended Data Fig. 7c,d), as indicated by a stratum-adjusted correlation coefficient of more than 0.98 (Extended Data Fig. 7b). Consequently, all analyses were performed using the combined data from the replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.