Shaker Kv channel expression
To produce the Shaker Kv channel for cryo-EM, the full-length gene was cloned into the pEG vector in which eGFP was substituted with mVenus56 to produce constructs with the mVenus tag on either the C or N terminus and a TEV protease site between mVenus and the channel. In addition, the full-length E12K/D13K Shaker Kv channel was generated by introducing mutations into the WT construct with a C-terminal mVenus tag. These constructs were expressed in tsA201 cells (Sigma-Aldrich) using the previously published baculovirus-mammalian expression system with a few minor modifications57. In brief, P1 virus was generated by transfecting Sf9 cells (Thermo-Fisher; approximately 2.5 million cells on a T25 flask with a vent cap) with 10 µg of fresh Bacmid using Cellfectin (Thermo-Fisher). After 4–5 days of incubation in a humidified 27 °C incubator, the cell culture media were collected by centrifugation (at 4,000g for 30 min), supplemented with 2% FBS and filtered through a 0.22-μm filter to harvest the P1 virus. To amplify the P1 virus, approximately 500 ml Sf9 cell cultures at a density of approximately 1 million cells per millilitre were infected with 1–200 μl of the virus and incubated in a 27 °C shaking incubator for 5 days. The cell culture media were then collected by centrifugation (at 5,000g for 30 min), supplemented with 2% FBS and filtered through 0.22-μm filter to harvest P2 virus. The P2 virus was protected from light using aluminum foil and stored at 4 °C until use. To express the Shaker channels, tsA201 cells at approximately 1.5 million cells per millilitre in Freestyle medium with 1% FBS were transduced with 5% (v/v) P2 virus and incubated at 37 °C in a CO2 incubator. To boost the protein expression, sodium butyrate (2 M stock in H2O) was added to 10 mM at approximately 16 h post-transduction. The culture was continued at 30 °C in a CO2 incubator for another 32 h, and the cells were harvested by centrifugation (at 5,000g for 30 min) and frozen at −80 °C until use.
Shaker Kv channel purification
Before extraction of the Shaker channels from tsA201 cells, membrane fractionation was carried out using a hypotonic solution and ultracentrifugation. In our initial trials (such as those in Fig. 1b–d), cells were first resuspended in a hypotonic solution (20 mM Tris pH 7.5 and 10 mM KCl) supplemented with a cOmplete protease inhibitor cocktail tablet using a Dounce homogenizer, incubated at 4 °C for approximately 30 min, and centrifuged at 6,000g for 10 min to remove cell debris. Once we realized there was considerable proteolysis of the N terminus, for EI Shaker, we increased protection from proteolysis by the addition of 1 µg ml−1 pepstatin, 1 µg ml−1 aprotinin, 1 µg ml−1 leupeptin, 0.5 µg ml−1 benzamidine, 0.1 µg ml−1 soy trypsin inhibitor and 1.5 mM phenylmethylsulfonyl fluoride (PMSF) to the lysis buffer before homogenizing and replenished again after homogenizing. In all instances, the supernatant was ultracentrifuged for 1 h (at 195,000g); membranes were collected and stored at −80 °C until use. To purify Shaker Kv channels, the fractionated membranes were resuspended in an extraction buffer (50 mM Tris pH 7.5, 150 mM KCl, 2 mM tris(2-carboxyethyl)phosphine hydrochloride (TCEP), 50 mM n-dodecyl-β-d-maltoside (DDM) and 5 mM cholesteryl hemisuccinate Tris salt (CHS) with the protease inhibitor mixture used above) and incubated for 1 h at 4 °C. The lysate was clarified by centrifugation (at 12,000g for 10 min) and incubated with CoTALON resins (Takara) at 4 °C for 1 h. The mixture was transferred to an empty disposable column (Econo-Pac, Bio-Rad) and the resin was washed with 10 column volume of buffer A (50 mM Tris pH 7.5, 500 mM KCl, 1 mM DDM, 0.1 mM CHS and 0.1 mg ml−1 porcine brain total lipid extract) with 20 mM imidazole before eluting bound proteins with buffer A with 250 mM imidazole. The eluate was concentrated using Amicon Ultra (Millipore; 100 kDa cut-off) to approximately 350–450 μl and loaded onto a Superose6 (Cytiva; 10/300) gel filtration column and separated with buffer (50 mM Tris pH 7.5, 150 mM KCl, 1 mM DDM and 0.1 mM CHS). All purification steps described above were carried out at 4 °C or on ice.
Lipid nanodisc reconstitution of the Shaker Kv channel
Lipid nanodisc reconstitution was performed following the previously published methods with minor modifications33. On the day of nanodisc reconstitution, the purified Shaker Kv channel obtained from gel filtration in detergent was concentrated to approximately 1–3 mg ml−1 and incubated with histidine-tagged MSP1E3D1 and 3:1:1 mixture of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (POPG) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) for 30 min at room temperature. The mixture was transferred to a tube with SM-2 Biobeads (approximately 30–50-fold of detergent, w/w) and incubated at room temperature for approximately 3 h with rotation in the presence of TEV protease (prepared in-house) and 2 mM TCEP to remove either N-terminal or C-terminal fusion protein including polyhistidine and the mVenus tag. The reconstituted protein was loaded onto a Superose6 column (10/300) and separated using 20 mM Tris pH 7.5, 4 mM KCl and 46 mM NaCl buffer at 4 °C. The success of nanodisc reconstitution was confirmed by collecting separated fractions and running SDS–PAGE to verify the presence of Shaker Kv and MSP1E3D1 bands at a similar ratio. Typically, optimal reconstitution required the incubation of a 1:10:200 or 1:10:400 molar ratio of tetrameric Shaker Kv, MSP1E3D1 and the lipid mixture, respectively. The sample in the nanodisc was concentrated to 2.5–4 mg ml−1.
Mass spectrometry analysis
Protein samples for the Shaker Kv channel were reconstituted in lipid nanodisc and run on SDS–PAGE. Excised bands for the Shaker proteins were reduced with 5 mM TCEP (Sigma-Aldrich), alkylated with 5 mM N-ethylmaleimide (NEM; Sigma-Aldrich) and digested with trypsin (Promega). Tryptic peptides were extracted then desalted before being injected into a nano-liquid chromatography with tandem mass spectrometry (nano-LC–MS/MS) system. For WT Shaker, LC–MS/MS data acquisition was carried out on an Orbitrap Ascend tribrid mass spectrometer (Thermo Scientific) with an EASY-Spray Ion Sources (Thermo Scientific) and coupled to a Vanquish Neo HPLC (Thermo Scientific). Of digests, 0.1–1 µg was loaded and desalted with an Acclaim PepMap 100 trapping column (75 µm × 2 cm; Thermo Scientific). Peptides were separated on an ES902 Easy-Spray column (75-μm inner diameter, 25 cm in length and 3 μm C18 beads; Thermo Scientific). The composition of mobile phase A (MPA) was 0.1% formic acid (Millipore-Sigma) in LC–MS grade water. The mobile phase B (MPB) was 0.1% formic acid in LC–MS grade acetonitrile (Millipore-Sigma). MPB was increased from 4% to 20% in 38 min. The flow rate was set at 300 nl min−1. Mass spectrometers were operated in data-dependent mode. The resolution of the survey scan was set at 120,000. The m/z range for the MS scan was 350–1,400. MS/MS scans were performed in the ion trap using higher-energy collisional dissociation (HCD) with the collision energy fixed at 30%. The minimum signal intensity required to trigger MS/MS scan was 1 × 104. MS1 scan was performed every 2 s. As many MS2 scans allowed were acquired within the MS1 scan cycle.
For GT Shaker samples and EI Shaker samples, an Ultimate 3000 HPLC (Thermo Scientific) and an Orbitrap Fusion Lumos Tribrid Mass Spectrometer (Thermo Scientific) were used for data acquisition. The LC–MS/MS method used was very similar to the one described above. The small differences included: MPB was increased from 5% to 22% in 39 min; the m/z range for MS scan was 375–1,500; for GT Shaker, peptides were fragmented with an electron-transfer/higher-energy collision dissociation (EThcD) method.
Raw data were processed with Mascot Distiller and searched with Mascot Daemon software (Matrix Science). The search was performed against the house-built database containing the WT Shaker and EI Shaker sequences. The mass tolerances for precursor and fragment were set to 5 ppm and 0.5 Da, respectively. SemiTrypsin was used with up to three missed cleavages allowed. NEM on cysteines was set as fixed modification. Variable modifications included oxidation (M), Met-loss (protein N-term), Met loss + acetyl (protein N-term) and acetyl (N-term). The search results were filtered by a false discovery rate of 1% at the protein level. Peptides detected by database search were manually curated.
Reconstitution of Shaker Kv channels into liposomes for injection into oocytes
On the day of reconstitution, the Shaker Kv channel purified by Superose6 in detergent was concentrated to 1–3 mg ml−1 and incubated for 30 min at room temperature with a solution containing 20 mg ml−1 of a 3:1:1 mixture of POPC, POPG and POPE where the mass ratio of protein to lipid was 1:10. The mixture was transferred to a tube with SM-2 Biobeads (approximately 30–50-fold of detergent, w/w) and incubated at room temperature for approximately 3 h with rotation either in the absence or presence of TEV protease (prepared in-house) with 2 mM TCEP to remove either N-terminal or C-terminal fusion protein including polyhistidine and mVenus tags. Biobeads were allowed to settle, and the resulting solution used for injection into oocytes. The final concentration of protein in liposome was 1 mg ml−1.
Cryo-EM sample preparation and data acquisition
For C-terminal mVenus-tagged Shaker, concentrated sample in nanodiscs (3 µl), with or without the addition of 1.5 mM fluorinated Fos-choline-8 (Anatrace) were applied to glow-discharged Quantifoil grids (R1.2/1.3 Cu 300 mesh). For GT Shaker, 3 µl sample in nanodiscs was applied to glow-discharged Quantifoil grids (R1.2/1.3 Cu 300 mesh). For the full-length E12K/D13K AcA-EI Shaker construct, the sample was applied to glow-discharged Ultrafoil grids (R1.2/1.3 Au 300 mesh). For the full-length E12K/D13K AcA-EI Shaker sample with 1 mM free N-terminal peptide, the peptide was incubated with protein for 30 min before grid preparation. Acetylated free N-terminal peptide (Ac-AAVAGLYGLGKKRQHRKKQ; molecular weight 2,151 Da) was synthesized by GenScript. After incubation, 3 µl sample was applied to glow-discharged Ultrafoil grids (R1.2/1.3 Au 300 mesh). The grids were blotted for 2.5 s, with a blot force of 4, at 100% humidity at 16 °C using a FEI Vitrobot Mark IV (Thermo Fisher), followed by plunging into liquid ethane cooled by liquid nitrogen.
Images were acquired using an FEI Titan Krios equipped with a Gatan LS image energy filter (slit width of 20 eV) operating at 300 kV. A Gatan K3 Summit direct electron detector was used to record movies in super-resolution mode with a nominal magnification of ×105,000, resulting in a calibrated pixel size of 0.415 Å per pixel. The typical defocus values ranged from −0.5 to −2.0 µm. Exposures of 2 s were dose fractionated into 40 frames, resulting in a total dose of 52 e− Å−2. Movies were recorded using the automated acquisition program SerialEM58.
Image processing
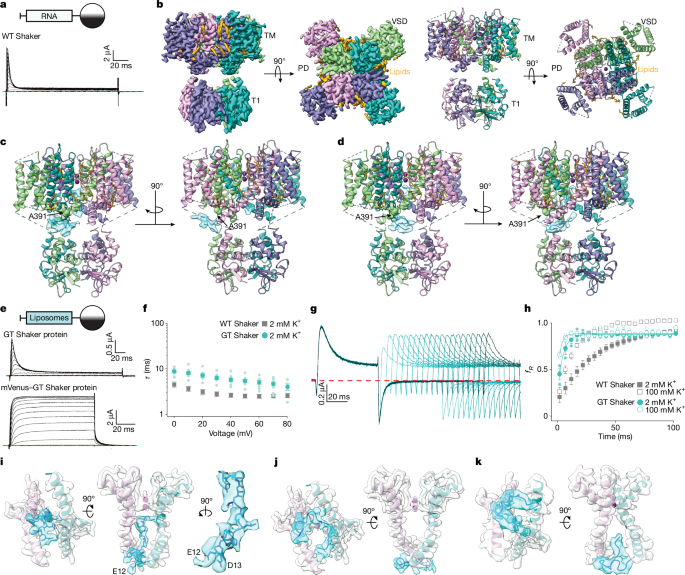
All processing was completed in RELION59 and cryoSPARC60. In general, the beam-induced sample motion between frames of each dose-fractionated micrograph was corrected and binned by 2 using MotionCor2 (ref. 61) or Patch Motion Correction, and contrast transfer function (CTF) estimation was performed using CTFFIND4 (ref. 62) or Patch CTF estimation. Micrographs were selected and those with outliers removed based on defocus value and astigmatism, as well as low resolution (more than 5 Å) reported by CTF estimation. The initial set of particles from subset micrographs were picked using Blob picker or Gautomatch (https://www2.mrc-lmb.cam.ac.uk/research/locally-developed-software/zhang-software/#gauto), followed by reference-free 2D classification. The good classes were then used as template to pick particles from all selected micrographs using a different program (including Gautomatch, Topaz pick or Template Picker). The best particles were selected iteratively by selecting the 2D class averages and 3D reconstructions (using C1 or C4 symmetry) that had interpretable structural features. After performing non-uniform refinement in either C1 or C4 symmetry, the unsharpened map of the C-terminal mVenus-tagged Shaker exhibited significantly weaker density in the internal pore than GT Shaker. Therefore, we used GT Shaker as the model for subsequent data processing.
For GT Shaker, the best particles were aligned using 3D autorefine in C4 symmetry in RELION. To further classify the particles, the particles were expanded from C4 to C1 symmetry. These particles were submitted to 3D classification for 10 classes without alignment. A cylindrical mask covering the pore region of two neighbouring monomers and the corresponding chamber between the transmembrane and T1 domains was used for 3D classification. Among 10 classes, one good class clearly showed L-shape density extending from the chamber to the internal pore, and this L-shape density was used to generate a mask for further classification (Extended Data Fig. 3).
To obtain a high-resolution map of the internal pore and chamber, the best particles were imported to cryoSPARC and subjected to NU Refinement with applied C4 symmetry. The 1.4 million aligned particles were submitted to 3D classification using the principal component analysis (PCA) mode in C1 symmetry with L-shape mask (10 classes, a filter resolution of 3 Å, online expectation-maximization [O-EM] batch size of 8,000 and O-EM learning rate init of 0.6–0.8) for 3 rounds. Four classes (1.1 million particles), exhibiting L-shape density within a 90° rotation from one to the next, were applied to Align 3D Maps. To separate different conformations of the N-terminal peptide within the internal pore (for example, Extended Data Fig. 6c), the aligned particles (1.1 million) were then further classified by changing the O-EM learning rate init to 0.0001 and the F-EM iters to 100. The best class (141,800) was selected and subjected to final local refinement with full-length protein mask in C1 symmetry. The final reconstruction was reported at 2.94 Å (Extended Data Fig. 3).
The other classes (424,000) showed density occupying in the chamber without extending into the internal pore. Further classification was applied for these particles, which generated two different classes, following local refinement in C1 symmetry. The resolutions of final constructs were reported at 2.79 Å and 3.01 Å (Extended Data Fig. 3).
During data processing, we noticed that the resolution of the T1 domain is much lower than the transmembrane domain. To get a high-resolution map of the T1 domain, the density of the T1 domain was obtained by subtracting the transmembrane region from the full-length protein. The subtracted particles were then submitted to 3D classification using PCA mode with a T1 domain mask in C1 symmetry by changing the filter resolution to 6 Å. All the classes were aligned using Align 3D Maps and followed by local refinement in C4 symmetry. The final reconstruction for the T1 domain was reported at 2.31 Å (Extended Data Fig. 3).
The other datasets for Shaker with a C-terminal mVenus tag (Supplementary Fig. 1), and the AcA-EI Shaker construct in the absence (Supplementary Fig. 2) or presence (Supplementary Fig. 3) of additional N-terminal peptide were processed using a method similar to GT Shaker.
Model building and structure refinement
Model building was first carried out by manually fitting the transmembrane domain of Shaker (Protein Data Bank ID: 8TEO) and the T1 domain generated by AlphaFold3 (ref. 63) into the electron microscopy density map using UCSF Chimera64. The model was then manually built in Coot65 and refined using real_space_ refine in PHENIX66 with secondary structure and geometry restraints. The final model was evaluated by comprehensive validation in PHENIX. Structural figures were generated using PyMOL (https://pymol.org/2/support.html), UCSF Chimera64 and UCSF ChimeraX67.
Electrophysiological recordings
For electrophysiological recordings, the full-length Shaker Kv channel cDNAs were cloned into the pGEM-HE vector68. Mutagenesis was performed by the QuikChange Lightning Kit (Agilent) using the full-length channel unless otherwise indicated. The DNA sequence of all constructs and mutants was confirmed by automated DNA sequencing. cRNA was synthesized using the T7 polymerase (mMessage mMachine Kit, Ambion) after linearizing with Nhe-I (NEB).
Oocytes (stage V–VI) from female Xenopus laevis frogs (approximately 1–2 years of age from Xenopus I) were removed surgically and incubated for 1 h at 19 °C in a solution containing: NaCl (82.5 mM), KCl (2.5 mM), MgCl2 (1 mM), HEPES (5 mM), pH 7.6, with NaOH and collagenase type II (2 mg ml−1; Worthington Biochemical). The animal care and experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke (animal protocol number 1253). Defolliculated oocytes were injected with cRNA or liposomes containing reconstituted Shaker Kv channels and incubated at 16 °C in a solution containing: NaCl (96 mM), KCl (2 mM), MgCl2 (1 mM), CaCl2 (1.8 mM), HEPES (5 mM), pH 7.6 (with NaOH), and gentamicin (50 mg ml−1; GIBCO-BRL) for 24–72 h before electrophysiological recording. Oocyte membrane voltage was controlled by an OC-725C oocyte clamp (Warner Instruments) and controlled using pClamp (10.7). Data were filtered at 1 kHz (8-pole Bessel) and digitized at 5–10 kHz. Microelectrode resistances ranged from 0.2 to 0.6 MΩ when filled with 3 M KCl. Oocytes were studied in 150 µl recording chambers that were perfused continuously with an extracellular solution containing: NaCl (98 mM), KCl (2 mM), MgCl2 (1 mM), CaCl2 (0.3 mM) and HEPES (5 mM), pH 7.6, with NaOH. When other external K+ concentrations were used, NaCl was replaced with KCl. Most experiments were undertaken in lower external K+ to approximate physiological conditions, and elevated external K+ was used in some experiments where inward tail currents were measured to compare the gating properties of different mutants. All experiments were done using a continuous flowing external solution and were carried out at room temperature (22 °C). Leak and background conductances were subtracted for tail current measurements by arithmetically deducting the end of the tail pulse of each analysed trace. In most instances, Kv channel currents shown are non-subtracted, but where indicated, a P/−4 leak subtraction protocol was used.
The Boltzmann equation was fit to G–V relations to obtain the V1/2 and z values according to
$$\frac{I}{{I}_{\max }}=\left(1+{e}^{-zF(V-{V}_{1/2})/RT}\right)$$
where z is the equivalent charge, V1/2 is the half-activation voltage, F is Faraday’s constant, R is the gas constant and T is temperature in Kelvin. Time constants of inactivation were obtained by fitting a single or double exponential function to the decay of currents using the following equation:
$$f(t)=\mathop{\sum }\limits_{i=0}^{n}{A}_{i}{e}^{-t/{\tau }_{i}}+C$$
where A is the amplitude and τ is the time constant. All analyses of electrophysiological data were done using Origin 2023b.
Sample size
Statistical methods were not used to determine sample size. Sample size for cryo-EM studies was determined by availability of microscope time and to ensure sufficient resolution for model building. Sample size for electrophysiological studies was determined empirically by comparing individual measurements with population data obtained under differing conditions until convincing differences or lack thereof were evident. For all electrophysiological experiments, n values represent the number of oocytes studied between 2 and 10 different frogs (indicated as independent experiments).
Data exclusions
For electrophysiological experiments, exploratory experiments were undertaken with varying ionic conditions and voltage-clamp protocols to define ideal conditions for measurements reported in this study. Although these preliminary experiments are consistent with the results that we report, they were not included in our analysis due to varying experimental conditions (for example, solution composition and voltage protocols). Once ideal conditions were identified, electrophysiological data were collected for control and mutant constructs until convincing trends in population datasets were obtained. Individual cells were also excluded if cells exhibited excessive initial leak currents at the holding voltage (more than 0.5 µA), if currents arising from expressed channels were too small (less than 0.5 µA), making it difficult to distinguish the activity of expressed channels from endogenous channels, or if currents arising from expressed channels were too large, resulting in substantial voltage errors or changes in the concentration of ions in either intracellular or extracellular solutions.
Randomization and blinding
Randomization and blinding were not used in this study. The effects of different conditions or mutations on Shaker Kv channels heterologously expressed in individual cells was either unambiguously robust or clearly indistinguishable from control conditions.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.