Mice
All mice were housed in animal facilities at Cold Spring Harbor Laboratory (CSHL), with food and water available ad libitum and in a 12 h–12 h light–dark regime, and were used at 7–14 weeks of age. BALB/c mice (BALB/cAnNCrl) were obtained from Charles River. C57BL/6 mice were obtained from The Jackson Laboratory. LysM–eGFP mice were originally provided by M. Looney (University of California, San Francisco). MMTV-PyMT mice were purchased from The Jackson Laboratory. The C3(1)-Tag mice were originally obtained from The Jackson Laboratory and bred in-house. PAD4ΔN mice were generated in-house by crossing Mrp8Cre mice (B6.Cg-Tg(S100A8-cre,-eGFP)1Ilw/J) with Padi4fl/fl mice and were compared with Cre-negative Padi4fl/fl littermates. NSG mice were purchased from The Jackson Laboratory. Neutropenic mice were generated in-house by crossing Lyz2Cre mice with Mcl1fl/fl mice, both purchased from The Jackson Laboratory. All mice were acclimated to the animal housing facility for at least 1 week before initiating experiments. No specific randomization method was followed in this study. No statistical methods were used to predetermine sample size: they were determined based on prior experience or pilot experiments performed in the Egeblad lab. Allocation of mice to groups ensured similar distribution of age and gender. Unless especified in the corresponding method, no specific blinding method was used.
All procedures were approved by the CSHL Institutional Animal Care and Use Committee and were conducted in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals.
Tumour models
PyMT cancer cells for orthotopic implantation were isolated from the primary tumours of MMTV-PyMT mice (C57BL/6 background). Tumours were mechanically dissociated and digested for 1 h with collagenase/hyaluronidase (10X, StemCell Technologies) containing DNase I (2 U ml−1; Roche) in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 5% fetal bovine serum (FBS) (VWR Life Science Seradigm). Debris was removed by pulse centrifugation in Hanks’ balanced salt solution (HBSS) supplemented with 5% FBS. Purified carcinoma organoids were further dissociated into a single-cell suspension in 0.05% trypsin with 0.1% EDTA supplemented with 2 U ml−1 of DNase I for 2–3 min. Single cancer cells were isolated by passing through a 100-µm cell strainer (BD Biosciences), washed with PBS, and immediately injected into the fourth mammary fat pads of anaesthetized C56BL/6 mice (2 × 105 cells per fat pad in 20 ml 1:1 PBS:Matrigel) to obtain the transplanted PyMT tumours. For the 4T1 tumour model, 5 × 104 4T1 cells were transplanted into the fourth mammary fat pads of anaesthetized BALB/c mice. For the LLC model, 1 × 105 LCC cells were subcutaneously injected into the shaved flanks of anaesthetized C57BL/6 mice. The C3(1)-Tag tumour model is a spontaneous genetic model in the FVB/n background that develops tumours that were analysed when the mice reached 22–26 weeks of age. Mice were bred and monitored for tumours. All cell lines were tested negative for Mycoplasma, as well as Corynebacterium, Ectromelia, EDIM, Hantaan, K virus, LCMV, LDEV, MAC1, MAV2, mCMV, MHV, MNV, MTV, MKPV, MVM, MPV, Polyoma, PVM, REO3, Sendai and TMEV.
TdTomato-expressing LLC tumour model
We generated tdTomato-expressing tumour cells using the third-generation lentivector pLenti6-EF1a-tdTomato-IRES-Blasti (provided by D. Plenker). After transduction, cells were selected with blasticidin, and tdTomato expression was confirmed by confocal microscopy.
Cxcl1-knockout 4T1 cells
Cxcl1 gene editing in 4T1 cells was done using a third-generation lentivector pLenti6-EF1a-Cas9-blasticidin-U6-guide RNA (provided by T. Zillinger). Single guide RNAs (sgRNAs) were designed using the E-CRISP design tool provided by the German Cancer Research Center and introduced into pLenti6-EF1a-Cas9-blasticidin-U6-guide RNA by Gibson assembly using the following sgRNA sequences: sg1 Cxcl1 – GCGCTGCACAGAGAAGCGAG, sg2 Cxcl1 – GCAGAGGTGTCCCCAAGTAA. Empty vector controls were transduced with the lentivector. After transduction of 4T1 cells and selection with blasticidin (5 µg ml−1), knockout cells were verified by Sanger sequencing. To confirm the depletion, we checked CXCL1 levels in culture supernatants using the Mouse CXCL1/KC DuoSet ELISA from R&D Systems (DY453) according to the manufacturer’s instructions.
dnTGFβR2-expressing 4T1 cells
dnTGFβR2-expressing 4T1 tumour cells were generated using the lentivector pLenti-CMV-Blast-DNTGFBR2-HA, which was from G.-P. Dotto27 (Addgene plasmid #130888) or a pLenti-CMV-Blast-DEST backbone for the control 4T1 cells. After transduction, cells were selected with blasticidin.
Flow cytometry and cell sorting
Flow cytometric analyses were performed using a Fortessa Analyzer (BD Biosciences) using BD Diva Software v8. The analysis was performed using FlowJo v10 (Tree Star Inc.). Cell sorting experiments were performed using a fluorescence-activated cell sorting (FACS) Aria cell sorter (BD Biosciences). All analyses were conducted at the Flow Cytometry Shared Resource at CSHL. Absolute quantification was done using Trucount absolute counting beads (340334, BD Biosciences) according to the manufacturer’s instructions.
Neutrophils were isolated from blood by FACS. In brief, blood was drawn into EDTA-coated tubes, red blood cells were lysed in ammonium–chloride–potassium (ACK; A1049201, Gibco) lysis buffer (Thermo Fisher), and blood cells were stained with antibodies to Ly6G, Ly6C and CD11b (Supplementary Fig. 1, immune panel). Immediately before flow cytometric analysis, DAPI was added to the cells. Then, DAPI−, CD11b+ and Ly6GHigh neutrophils were sorted. For some experiments, from this neutrophil population, Ly6CHigh and Ly6CLow neutrophils were sorted.
Cytometric analyses of blood and tissues from naive and tumour-bearing mice were performed as previously reported13. In brief, tissues (tumour, bone marrow, liver, lung and spleen) were extracted, kept in cold PBS (except liver, kept at room temperature in HBSS) and processed immediately upon collecting all samples. The lungs and tumours were digested in HBSS with liberase (1 U ml−1, Roche) and DNase I (1 mU ml−1, Sigma) for 30 min at 37 °C. The bone marrow and spleen were mechanically dissociated to prepare single-cell suspensions by flushing and straining, respectively. Leukocytes in the liver were enriched by centrifugation at 800g for 30 min, using a 36% Percoll (GE Healthcare; diluted in HBSS) gradient. Blood counts were analysed in an automated haemocytometer, and red blood cells were lysed in ACK hypotonic buffer. Single-cell suspensions from all tissues were incubated with fluorescently conjugated antibodies from the haematopoietic panel (bone marrow and spleen) or from the immune panel (all tissues). See Supplementary Fig. 1 for antibodies used for flow cytometry. Unbiased flow cytometric analysis tools54 were used for the data on Extended Data Fig. 9d–h.
Ex vivo NET formation assays with mouse neutrophils
Neutrophils were sorted as described above, and 4 × 104 neutrophils were plated in serum-free RPMI medium on poly-l-lysine-covered 8-well μ-slides (Ibidi), then left for 30 min at 37 °C in a cell culture incubator to adhere. Cells were plated in a drop of medium in the centre of the well to enhance their adhesion to the central area of the well and to avoid their deposition at the edges. Cells were subsequently incubated for 2 h with 100 nM PMA or vehicle. Cells were then fixed using 4% paraformaldehyde (PFA) in PBS for 10 min; blocked and permeabilized with PBS containing 0.1% Triton X-100, 25% FBS and 5% bovine serum albumin (BSA); and stained with antibodies to citH3 (Abcam) and MPO (R&D Systems) at 1:200 dilution in blocking buffer at 4 °C overnight. Then, the cells were washed and stained with secondary antibodies: donkey anti-goat AF647 (A21447, Invitrogen) and donkey anti-rabbit AF568 (A10042, Invitrogen) at 1:400 and counterstained with DAPI (1:1,000) for 2 h at room temperature. Z-stack images were acquired with a SP8 microscope (Leica) and analysed using Imaris (Bitplane) or custom-made ImageJ macros (see the section ‘Code availability’) to identify NETs (defined as triple co-localized MPO+, citH3+ and DNA (DAPI)+ and therefore citrullinated NETs).
Whole-mount immunostaining and tissue clearing
Mice were euthanized with CO2, and their tissues were immediately excised and submerged in 4% PFA in PBS and fixed at 4 °C overnight. After three washes with PBS for 1 h each at room temperature, the tissues were permeabilized in methanol (MetOH) gradients in PBS (PBS > MetOH 50% > MetOH 80% > MetOH 100%, for 30 min in each solution). Then, the tissues were bleached with Dent’s bleach (15% H2O2 and 16.7% dimethyl sulfoxide (DMSO) in MetOH) for 1 h at room temperature and rehydrated through descending methanol gradients in PBS (MetOH 80% > MetOH 50% > PBS, 30 min in each solution). Then, the tissues were incubated with a blocking buffer containing PBS with 0.3% Triton X-100, 0.2% BSA, 5% DMSO, 0.1% azide and 25% FBS overnight at 4 °C with shaking. Afterwards, the tissues were stained with primary antibodies (Supplementary Fig. 2) at 1:200 in blocking buffer for 3 days at 4 °C with shaking. After washing for 24 h in washing buffer (PBS with 0.2% Triton X-100 and 3% NaCl), the tissues were stained with secondary antibodies (Supplementary Fig. 2) at 1:400 for 3 days at 4 °C with shaking. Then, the tissues were washed for 24 h in washing buffer and thereafter dehydrated in MetOH gradients in dH20 using glass containers (MetOH 50% > MetOH 70% > MetOH 90% > 3× MetOH 100%, 30 min for each step). The tissues were then cleared for 30 min in 50% MetOH and 50% benzyl alcohol, benzyl benzoate (BABB, mixed 1:2) and for 1 h in 100% BABB and, finally, imaged on an SP8 microscope (Leica, typical Z-depths of 300–500 µm). Quantification was performed with Imaris software (Bitplane).
In some cases, when we needed to preserve endogenous fluorescence, we changed our clearing procedure and used clear, unobstructed brain imaging cocktails and computational analysis (CUBIC), a different tissue clearing protocol technique that maintains the endogenous fluorescence from fluorescent proteins. Tissues were excised and fixed as stated above, and then soaked in CUBIC-I solution in a 15-ml conical tube. CUBIC-I was prepared by mixing 108 ml of ddH2O with 75 g of urea (U5128, Sigma), 75 g of N,N,N′,N′-tetrakis(2-hydroxypropyl)ethylenediamine (122262, Sigma) and 42 ml of Triton X-100 (X100, Sigma). Samples were maintained at 37 °C on a shaker for 7 days, with the media changed every other day or until clear. The samples were washed in PBS and then blocked and stained as described above for the BABB protocol, except no methanol and no DMSO were used. Once the staining was complete, the samples were placed back in CUBIC-I to clear and then were counterstained for DAPI in CUBIC-I (1:1,000) for 24 h and washed in CUBIC-I overnight. Images were acquired and analysed as described above.
Slide immunofluorescence staining
Tissues were sectioned at the CSHL Animal and Tissue Imaging Shared Resource. To stain formalin-fixed paraffin-embedded (FFPE) slides, paraffin sections were first deparaffinized and rehydrated, and antigen retrieval was carried out by boiling slides in Tris EDTA buffer (10 mM Tris base and 1 mM EDTA, pH 9.0) for 10 min in a pressure cooker. Then, the tissues were incubated with a blocking buffer containing PBS with 0.1% Triton X-100 and 0.2% BSA for 30 min at room temperature. The tissues were incubated with the corresponding primary antibodies (see Supplementary Fig. 2 for a list of antibodies used in this study) overnight at 4 °C. The next day, the samples were washed three times in PBS and stained with the corresponding secondary antibodies for 2 h at room temperature. Then, the samples were washed, counterstained with DAPI and mounted with antifade medium (ProLong Gold, P10144, Thermo Fisher). The 3D9 antibody to stain NETs in human tissues14 was provided by A. Zychlinsky.
Perfused or unperfused vessel identification
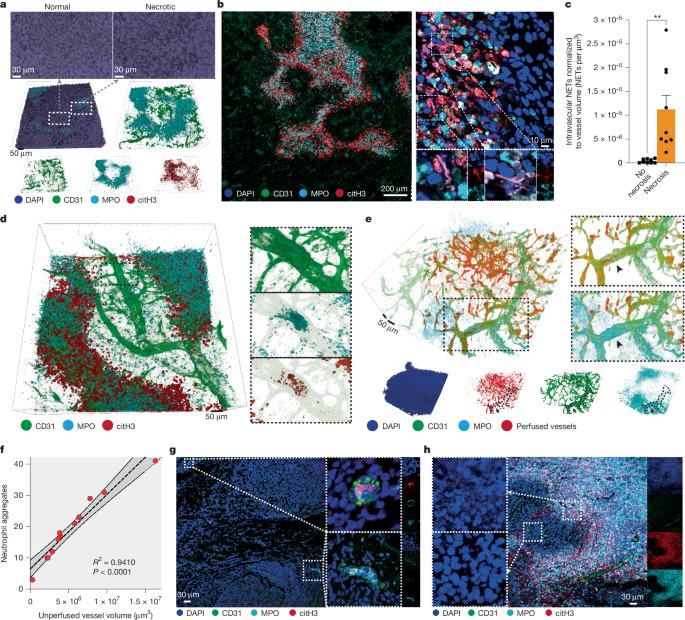
To visualize vessels perfused in vivo, we injected 50 μg of rhodamine-conjugated Griffonia simplicifolia lectin (RL-1102, Vector Laboratories) i.v. in 100 μl of sterile PBS for 3 h and again 10 min before euthanasia. Then, the tissues were immediately fixed, submerged in 4% PFA in PBS overnight and cleared as stated above. The vascular-specific lectin staining was independently confirmed by i.v. injecting 10 μg PE-conjugated vascular endothelial (VE)-cadherin (138106, BioLegend) in sterile PBS.
Immunofluorescence image quantification
Intravascular NETs were quantified in Bitplane Imaris. In brief, using the CD31 channel, a surface was generated that contained all of the vasculature and was used to mask the outside in the rest of the channels to quantify only intravascular events. Then, the triple colocalization of DNA, citH3 and MPO was used as the criterion to detect and quantify the NETs using Imaris spots.
To quantify the correlation between neutrophil aggregates and non-perfused vessels, we also used Imaris. In brief, a surface of perfused vessels (using i.v. injected lectin) and other of non-perfused vessels (CD31+lectin–) was generated. Then, the data type was set to 32 bit (float), and the distance transformation was calculated for both surfaces (A = perfused and B = non-perfused). Then, a new channel was generated using the formula A-B that yielded the intermediate regions (transition zones) between perfused and unperfused vessels. This new channel was used to generate a new surface (C = transition zones) that was used to mask the neutrophil channel (outside to zero). Finally, the number of neutrophil aggregates was calculated using the surfaces tool on that channel, with a minimum number of voxels of 100.
E-cadherin, vimentin and TGFβ in the slides were quantified using Imaris. In brief, a region of interest (ROI) was defined in perinecrotic and non-perinecrotic areas. Perinecrotic areas were defined as regions adjacent to necrotic areas up to 400 µm from the edge of necrosis (evaluated using nuclear morphology). Then, cells were detected using the DAPI signal, with a threshold (area over 200 μm2) to avoid smaller immune cells and only detect cancer cells, using the surfaces tool. Then, the per-cell intensities of the different channels were exported and used for quantification.
Imaging mass cytometry
Slides were cut from the FFPE blocks onto Superfrost Plus slides (Fisher Scientific). The slides were first baked for 2 h at 60 °C, dewaxed in xylene wash for 20 min and rehydrated in an alcohol gradient (100%, 95%, 80% and 70% EtOH in Maxpar water (Standard BioTools) for 5 min each). The slides were then washed with Maxpar Water for 5 min, then incubated in an Antigen Retrieval Agent (Cell Conditioning Solution CC1) at a temperature of 105 °C for 1 h. Slides were blocked with 3% BSA in Maxpar PBS for 45 min at room temperature in a hydration chamber. Selected antibodies were conjugated in-house, diluted to a concentration ranging from 0.25 mg ml−1 to 0.5 mg ml−1, then aliquoted for use. The slides were stained with the final antibody cocktail (Supplementary Fig. 3) overnight at 4 °C. Slides were subsequently washed with Triton-X100 in Maxpar PBS for 16 min, then washed in Maxpar PBS for 16 min. For DNA labelling, Cell-ID Intercalator-Ir (Fluidigm) was diluted at 1:400 in Maxpar PBS and used to stain for 30 min at room temperature in a hydration chamber. As a tissue counterstain, ruthenium tetroxide 0.5% stabilized aqueous solution (Polysciences) was diluted at 1:2,000 in Maxpar PBS and used for staining for 3 min. A final wash was performed in Maxpar Water for 5 min. Images were acquired with a Hyperion Imaging System (Standard BioTools) at the Sydney Kimmel Comprehensive Cancer Center Flow/Mass Cytometry and Technology Development Shared Resources. Following acquisition, stacks of multilayered ome.tiff images were exported, and representative images were generated utilizing MCD Viewer (Standard BioTools). Further analysis was performed using QuPath.
Intraluminal extracellular matrix component exposition assay
To visualize intraluminal exposition of extracellular matrix components in vivo, we i.v. injected 10 μg of antibodies against laminin (polyclonal anti-laminin antibody, ab11575, Abcam) into tumour-bearing mice. After 10 min of recirculation, mice were euthanized in CO2, and the tissues were immediately fixed, submerged in 4% PFA in PBS overnight and cleared as stated above (laminin was detected using a secondary antibody (Invitrogen; donkey anti-rabbit AF568) against the primary antibody; Supplementary Fig. 2).
Fibrin deposition assay
To analyse fibrin deposition in the primary tumour, we i.v. injected 15 mg kg−1 of fibrinogen conjugated to AF647 (F35200, Invitrogen) into tumour-bearing mice 24 h before euthanizing them and recovering the tissues. Then, the tissues were fixed and cleared using CUBIC-I (see the section ‘Whole-mount immunostaining and tissue clearing’).
Hypoxic region analysis
To visualize hypoxic regions in the tumours, we i.v. injected the tumour-bearing mice with 60 mg kg−1 of pimonidazole (part of the Hypoxyprobe-Biotin, HP10-1000kit, Hypoxyprobe) and waited 90 min, according to manufacturer’s instructions. After that, the mice were euthanized, and tissues were resected and immediately fixed using 4% PFA in PBS. Then, the samples were cleared (see the section ‘Whole-mount immunostaining and tissue clearing’) and stained using the reagents included in the kit.
Nuclear morphology analysis
To analyse nuclear morphology, sorted Ly6CHigh or Ly6CLow neutrophils were plated into an eight-well Ibidi μ-slide and allowed to adhere for 30 min at 37 °C. Then, the cells were fixed with 4% PFA in PBS for 10 min. The cells were stained for MPO and DAPI and imaged on an SP8 microscope (Leica). Finally, the number of nuclear lobes per cell was manually quantified using ImageJ (Fiji).
Chemotaxis assay
Chemotaxis assays were performed using Corning HTS transwell 24-well permeable supports (6.5 mm in diameter, pore size of 3 µm, Sigma) and Corning ultra-low attachment plates (Sigma). Either 600 µl of RPMI-1640 or RPMI-1640 containing 20 ng ml−1 CXCL1 (R&D Systems) was added to the lower chambers. The upper chambers were seeded with neutrophils (200 µl at 5 × 106 cells per millilitre) and incubated for 2 h at 37 °C in 5% CO2. The transmigrated neutrophils were collected from the lower chamber and analysed using flow cytometry.
Neutrophil–platelet and neutrophil–neutrophil aggregation experiments
To analyse the ability of neutrophils to interact with platelets, we quantified the ratio of neutrophil–platelet aggregates in circulation for Ly6CHigh, Ly6CInt and Ly6CLow neutrophils by flow cytometry. In brief, blood was collected in EDTA tubes, RBC lysed, and stained for Ly6G, Ly6C and CD41 as described above. Then, the proportion of Ly6G+ neutrophils with CD41+ signal was calculated.
To analyse the ability of our neutrophil populations to aggregate (neutrophil–neutrophil aggregation), Ly6CHigh, Ly6CInt and Ly6CLow neutrophils were sorted as previously described55,56. One million neutrophils of each category were stained with CellTracker Green CMFDA (Invitrogen) as per the manufacturer’s instructions, and then resuspended in 1 ml of RPMI and brought to the flow cytometer. Then, fMLP (Tocris) was added to the neutrophils to a final concentration of 1 μM, and each tube was immediately captured undisturbed for 6 min. Then, the percentage of neutrophil multiplets (based on Ly6G–AF647 and CellTracker Green signal) was calculated at 1-min intervals using FlowJo.
EdU labelling and half-life calculation
Pulse labelling was performed with 50 mg kg−1 of EdU (BaseClick) intraperitoneally (i.p.) injected. Then, the percentage of EdU+Ly6G+ cells was determined at days 1, 3 and 5 after injection by flow cytometry according to the manufacturer’s instructions. The half-life of neutrophils in circulation was calculated as follows: the decay constant λ was calculated from the formula: Nt = Np × e(−λ × Δt), where Nt is the percentage at time t, Np is the percentage at peak, and Δt is the difference in hours between Np and Nt. Then, using the decay constant λ, the half-life ‘h’ was calculated using the formula: 1 = 2 × e(−λ × h).
Neutrophil adhesion to fibrin
Uncoated Ibidi μ-slide (eight-well) plates were coated with fibrin overnight as previously described57. In brief, 0.6 μg ml−1 of mouse fibrinogen (Innovative Research) + 5 U ml−1 mouse thrombin (Innovative Research) were added to the uncoated slides and incubated at 37 °C overnight in a humidified incubator. The excess thrombin was then washed with RPMI, and the slides were allowed to dry for 15 min. RBC-lysed blood from 4T1 tumour-bearing mice was then added to the fibrin-coated wells and incubated at 37 °C in 5% CO2 for 30 min and subsequently washed with PBS to remove non-adherent cells. Then, the adhered cells were incubated with 0.05% trypsin for 1 min to detach them from the plate and were analysed by flow cytometry. The proportions of Ly6CHigh, Ly6CInt and Ly6CLow neutrophils in the input blood were then compared with the proportions in the fibrin-adhered cells. In some experiments, neutrophils were incubated with 10 μg ml−1 of anti-CD11c (BE0038, BioXcell) or control (BE0091, BioXcell) antibodies for 5 min before plating. In other experiments, Ly6CHigh, Ly6CInt and Ly6CLow neutrophils sorted from 4T1 tumour-bearing mice were plated onto fibrin-coated μ-slides and after 30 min of adhesion were incubated with 100 nM PMA to induce NET formation. After 2 h, plates were then fixed in 4% PFA for 10 min and stained for NETs as previously described.
CXCL1 treatment of isolated HSPCs in vitro
HSPCs were enriched from the bone marrow cells of BALB/c mice with the EasySep Mouse Hematopoietic Progenitor Cell Isolation Kit (19856, StemCell Technologies). These HSPCs were grown in SFEM medium (StemSpan Serum-Free Expansion Medium, 09650, StemCell Technologies), 10% FBS, 20 ng ml−1 stem cell factor (78064.1, StemCell Technologies), 20 ng ml−1 Flt3-L (250-31L, PeproTech), with or without 30 ng ml−1 CXCL1 (453-KC-010, R&D Systems). Cells were cultured for 11 days, and the medium was changed every 3–4 days. At the end point, the cells were counted and analysed by flow cytometry as previously stated.
Zymosan-induced peritonitis to measure extravasation efficiency
Mice were i.p. injected with 1 mg of Zymosan (Sigma). After 2 h, we took blood samples and obtained the peritoneal lavage for cytometric analyses and cell counts. We compared the percentage of neutrophils in the peritoneum versus blood from each donor to estimate the migration efficiencies of the neutrophils (percentage in peritoneum to percentage in blood). In some experiments, mice were i.v. injected with 50 µg of anti-Ly6C (InVivoMAb anti-mouse Ly6C, BE0203, BioXcell) or isotype control antibodies (InVivoMAb rat IgG2a isotype control, BE0089, BioXcell) immediately before the i.p. injection of Zymosan.
NET inhibition with disulfiram in the diet
Gamma-irradiated Purina rodent chow diet (#5053) repelleted (as control) and Purina chow diet (#5053) with 1 g of disulfiram per kg of diet (experimental) were purchased from Research Diets. Mice were fed with the control or experimental diet ad libitum starting upon tumour implantation and for the duration of the experiment. We previously confirmed the inhibition of NET formation in independent experiments using a model of acute lung injury, as well as in the tumour-bearing mice, and we confirmed this inhibition in all of the experimental groups.
Injectable drug treatments
Mice bearing 4T1 tumours were treated with 16 mg kg−1 of dipyridamole (Sigma) i.p. once a day, 0.5 mg kg−1 of tirofiban (Sigma) i.p. twice a day, or 300 U of DNase I (Roche) i.p. once a day starting 1 week after tumour implantation and until end point at day 28 after implantation. Control animals were treated with their respective vehicles (PBS for tirofiban and DNase I, sesame oil for dipyridamole).
ROS quantification
To analyse ROS levels in circulating neutrophils, RBC-lysed blood was stained with 5 mM dihydroethidium (Thermo Fisher) for 20 min and stained with antibodies to Ly6G and Ly6C (Supplementary Fig. 1) for cytometric analysis.
Phagocytosis assay
To analyse the ability of neutrophils to phagocytose in vivo, tumour-bearing mice were i.v. injected with 100 μg of Alexa Fluor 488-conjugated Zymosan BioParticles (Z23373, Invitrogen). Two hours later, blood was withdrawn and prepared for flow cytometry as described above, and the number of AF488-containing neutrophils was quantified. In parallel, some of the blood was used for direct imaging to confirm internalization using a Leica SP8 confocal microscope.
Experimental metastasis model
We performed an experimental metastasis assay to test the homing and growing ability of LLC cells independently of shedding from the primary tumour. First, 5 × 104 LLC cells were injected into the tail vein of PAD4WT or PAD4ΔN mice. Then, the mice were monitored for 2 weeks and euthanized at the end of the 2-week period. The lungs were excised and fixed overnight in 4% PFA in PBS and then sent to the CSHL Animal & Tissue Imaging Shared Resource for sectioning and H&E staining to quantify the metastatic burden (see the section ‘Quantification of H&E slides (metastasis and necrosis)’ below).
Quantification of H&E slides (metastasis and necrosis)
H&E lung or primary tumour slides from the different tumour-bearing mice were evaluated using QuPath software (v0.2.3) for quantitative pathology and bioimage analysis. The metastatic area in the lungs was calculated using multilayer perceptron (MLP-ANN) pixel classifier in QuPath trained to metastasis regions and compared with total lung area (excluding alveolar space). The necrotic area was similarly calculated using the MLP-ANN pixel classifier in QuPath trained to the necrotic regions and compared with the total tumour area (excluding adipose tissue, also trained in the classifier).
Intravital microscopy of the skin and laser injury
For intravital microscopy of the dermal microcirculation, the hair-removed (Veet) dorsal side of the ear of anaesthetized LysM–GFP mice was mounted on a custom-built support, and the ear was immersed in ultrasound gel and covered with a coverslip. Mice were then injected with antibodies to Ly6C (Ly6C–PE, BioLegend), and multiphoton imaging and laser ablation were performed as previously reported37 on an upright LaVision BioTec TrimScope (Bielefeld) equipped with a 16× water immersion objective, two lasers (Mai Tai and InSight, Spectra-Physics) and an optical parametric oscillator. The following Longpass Dichroic Beamsplitters (Chroma) were used to direct the signal towards photomultiplier tubes: T560LP, T665LPXXR and T495lxpr.
Intravital microscopy of LLC tumours
LysM–GFP mice were subcutaneously implanted with LLC tumours in the flank. Seven days after implantation, the mice were anaesthetized with inhaled isoflurane, the skin was carefully removed, and a transparent PDMS window was implanted as previously reported15. The mice were allowed to recover for 1 week, and then imaging was performed on the same LaVision system described above. To visualize neutrophil–platelet dynamics in the microvasculature in vivo, mice were injected with 0.5 μg of anti-CD41–PE antibodies (CD41–PE, BioLegend) and 50 μg of AF647-conjugated G. simplicifolia lectin to visualize blood vessels.
RNA-seq
Ly6CHigh and Ly6CLow neutrophils or TdTomato-expressing cancer cells from PAD4WT or PAD4ΔN mice were sorted as stated above. Then, total RNA was extracted using a Qiagen RNeasy Plus kit, according to the manufacturer’s instructions. We then measured the concentration of the total RNA using Qubit (Thermo Fisher) and an Agilent RNA 6000 Nano kit (5067-1511) to analyse its quality. Libraries were made using the Kapa mRNA Hyper prep protocol: the mRNA was captured with oligo-dT beads and then fragmented using heat and magnesium. The first cDNA strand was synthesized using random priming. Then, this step was followed by a combined second-strand synthesis and A-tailing, which converted the cDNA–RNA hybrid to double-stranded cDNA, incorporated dUTP into the second cDNA strand, and added dAMP to the 3′ ends of the resulting double-stranded cDNA. The next steps were Illumina adaptor ligation and then library amplification using high-fidelity, low-bias PCR. The strand marked with dUTP was not amplified, allowing strand-specific sequencing. As starting material, we used 100 ng of total RNA in 50 μl of DNase-free water. For fragmentation, we incubated the samples for 6 min at 85 °C (fragments of 300–400 bp). For PCR enrichment, we used 13 cycles. Quality control for the final libraries was done using Qubit (DNA HS assay) and Bioanalyzer (DNA HS assay), and then we pooled the samples and checked the final library using the Kapa qPCR. Sequencing was done using the Illumina NextSeq 500/550 Mid Output Kit v2.5 (approximately 24 million single-end 75-cycle reads for each sample).
Further analyses were performed using R 4.0.4 (‘Lost Library Book’) and Bioconductor 3.12. In brief, Gene Ontology terms were obtained with gProgileR, using a maximum P value of 0.05 and the false discovery rate (FDR) as the correction method. Lists of DESeq2-detected upregulated and downregulated genes (that is, the upregulated and downregulated gene signature of Ly6CLow neutrophils) were also analysed using gProgileR with the same settings. A reactome pathway analysis was performed using the enrichPathway function of the ReactomePA library, from the ENTREZ nomenclature (mapped with the mapIds function of the AnnotationDb library) of the same lists used for the Gene Ontology terms, with a q value and P value cut-off of 0.05 in both cases. Only pathways with an adjusted P value under 0.05 were kept. Volcano plots were represented using the EnhancedVolcano library, with a fold-change cut-off of 2 and a P value cut-off of 0.05. Reactome pathway clustering was performed using pathfinder: first, the run_pathfindR function was run on the reactome gene set using the same input lists as before. Then, we used the cluster_enriched_terms function to perform the actual clustering of the reactome pathways. Top genes were extracted with dplyr. The session used the following libraries: limma (3.46.0), edgeR (3.32.1), tximport (1.18.0), edgeR (3.32.1), sva (3.38.0), RColorBrewer (1.1-2), pheatmap (1.0.12), biomaRt (2.46.3), ggplot2 (3.3.3), gplots (3.1.1), ggfortify (0.4.11), NMF (0.23.0), cluster (2.1.1), fpc (2.2-9), plyr (1.8.6), dplyr (1.0.5), pvclust (2.2-0), ggrepel (0.9.1), amap (0.8-18), gProfileR (0.7.0), xtable (1.8-4), ggpubr (0.4.0), tidyr (1.1.3), DESeq2 (1.30.1), ReactomePA (1.34.0), stringr (1.4.0), Org.Hs.eg.db (3.12.0), pathfindR (1.6.1), CompGO (1.26), EnhancedVolcano (1.8.0) and GeneBook (1.0).
Computational analysis of cytometric data using FlowSOM
To analyse neutrophil heterogeneity using flow cytometry data, we used a computational analysis with the FlowSOM library using R 4.1.2 (‘Bid Hippie’). First, compensated data were cleaned (DAPI negative, single cells) and exported using FlowJo. Then, the data were loaded into R using the flowCore library, and all non-trivial, non-DAPI channels were used as marker columns. Then, a flowFrame was created, and from it, a FlowSOM object using the selected marker columns, using a 10 × 10 grid of clusters and automatic metaclustering. tSNE plots were computed using the Rtsne function. The final report was built using the FlowSOMmary function. The session used the following libraries: ggrepel (0.9.1), cytofkit2 (0.99.80), VGAM (1.1-6), reticulate (1.22), plyr (1.8.6), slingshot (2.2.0), TrajectoryUtils (1.2.0), princurve (2.1.6), uwot (0.1.11), Matrix (1.4-0), destiny (3.9.0), RColorBrewer (1.1-2), reshape2 (1.4.4), scales (1.1.1), dplyr (1.0.7), SingleCellExperiment (1.16.0), SummarizedExperiment (1.24.0), Biobase (2.54.0), GenomicRanges (1.46.1), GenomeInfoDb (1.30.1), IRanges (2.28.0), S4Vectors (0.32.3), BiocGenerics (0.40.0), MatrixGenerics (1.6.0), matrixStats (0.61.0), Rtsne (0.15), FlowSOM (2.2.0), igraph (1.2.10), flowCore (2.6.0), pheatmap (1.0.12), BiocManager (1.30.16), viridisLite (0.4.0) and ggplot2 (3.3.5).
scRNA-seq analyses of 4T1 and MMTV-PyMT tumours
We analysed the Gene Expression Omnibus (GEO) dataset GSE123366 for 4T1 (GSM3502134) and MMTV-PyMT (GSM3502136) cancer cells using R 4.1.2 (Bid Hippie). The datasets were subjected to quality control, and cells with mitochondrial transcripts above 10% were discarded (we used AnnotationHub to retrieve chromosomal localizations), as well as cells with a low number of detected features or low total counts (less than 10% of the mean). Then, counts were log normalized using the logNormCounts function, and both datasets were merged using the scMerge library. Cell annotation was performed using SingleR and the ImmGenData libraries. High-variable gene selection was performed with the getTopHVGs function, keeping all genes above the fit for the variance of the log-normalized expression values for each gene across all cells in the population, calculated with the modelGeneVar function. Principal component analysis (PCA) was then calculated using Scater, and elbow calculation using the PCAtools library was used to trim the principal components. The tSNE was calculated using the Scater library. Differential gene set activity for Gene Ontology terms between 4T1 and MMTV-PyMT cells was calculated for the Gene Ontology term aggregated data using the sumCountsAcrossFeatures function from the Scuttle library. Pseudobulk analyses were performed using DESeq2. Differentially expressed genes were defined as those with an adjusted P value under 0.05. Reactome pathway clustering was performed using pathfindr and the Reactome gene set. The session used the following libraries: DESeq2 (1.34.0), png (0.1-7), apeglm (1.16.0), reshape2 (1.4.4), magrittr (2.0), edgeR (3.36.0), Matrix.utils (0.9.8), enrichplot (1.14.1), ggpubr (0.4.0), GO.db (3.14.0), clusterProfiler (4.2.2), genesorteR (0.4.3), RColorBrewer (1.1-2), slingshot (2.2.0), TrajectoryUtils (1.2.0), princurve (2.1.6), scRNAseq (2.8.0), pathview (1.34.0), limma (3.50.0), dynamicTreeCut (1.63-1), dendextend (1.15.2), pheatmap (1.0.12), clustree (0.4.4), ggraph (2.0.5), msigdbr (7.4.1), kableExtra (1.3.4), scran (1.23.1), org.Mm.eg.db (3.14.0), AnnotationDbi (1.56.2), batchelor (1.10.0), dittoSeq (1.6.0), SingleR (1.8.1), scMerge (1.10.0), scater (1.22.0), scuttle (1.4.0), AnnotationHub (3.2.1), BiocFileCache (2.2.1), dbplyr (2.1.1), DropletUtils (1.14.2), RCurl (1.98-1.5), cowplot (1.1.1), scales (1.1.1), Matrix (1.4-0), forcats (0.5.1), stringr (1.4.0), dplyr (1.0.7), purrr (0.3.4), readr (2.1.2), tidyr (1.1.4), tibble (3.1.6), ggplot2 (3.3.5), tidyverse (1.3.1), SeuratObject (4.0.4), Seurat (4.1.0), SingleCellExperiment (1.16.0), SummarizedExperiment (1.24.0), GenomicRanges (1.46.1), GenomeInfoDb (1.30.1), IRanges (2.28.0), S4Vectors (0.32.3), MatrixGenerics (1.6.0), matrixStats (0.61.0), GEOquery (2.62.2), Biobase (2.54.0) and BiocGenerics (0.40.0).
scRNA-seq analyses of 4T1 and C3(1)-Tag tumours for hypoxic cells
We analysed the GEO dataset GSE123366 for 4T1 tumours (sample GSM3502134) and GEO data series GSE199515 for C3(1)-Tag tumours (samples GSM5974489 and GSM5974490). Quality control and annotation were performed as stated above. Only epithelial cells were kept for subsequent analysis. We calculated the Gene Ontology terms using the goana function of the limma library, keeping only those that were of the biological processes ontology, significant and not overly general (n ≤ 500). Then, using sumCountsAcrossFeatures, as stated above, we aggregated the data by Gene Ontology term and used the term ‘response to hypoxia’ (GO:0001666) to split the cells into two groups: hypoxic cells, that is, those expressing values higher than the median of the total population for the ‘response to hypoxia’ term, and normoxic cells, those expressing values lower than the median. PCA was then calculated using Scater and trimmed using the elbow calculation of the PCAtools library. tSNE was calculated using the Scater library. Marker genes upregulated in both categories were identified using the findMarkers function of the Scran library and the Wilcoxon rank-sum test, keeping those with an FDR under 0.05. Heatmaps of those marker genes were plotted using the plotMarkerHeat function of the genesorteR library. Category terms from these markers were calculated using the goana function as stated above, and Gene Ontology terms were plotted using ggplot2, showing only those with an adjusted P value under 0.0000001. Reactome pathways were calculated using the ReactomePA library, using the same gene marker lists, with a P and q value cut-off of 0.05, and keeping only those pathways with an adjusted P value under 0.05. The session used the following libraries: ReactomePA (1.38.0), pathfindR (1.6.3), pathfindR.data (1.1.2), CompGO (1.28.0), RDAVIDWebService (1.26.0), GOstats (2.60.0), Category (2.60.0), graph (1.72.0), DESeq2 (1.34.0), png (0.1-7), apeglm (1.16.0), reshape2 (1.4.4), magrittr (2.0.1), edgeR (3.36.0), Matrix.utils (0.9.8), enrichplot (1.14.1), ggpubr (0.4.0), GO.db (3.14.0), clusterProfiler (4.2.2), genesorteR (0.4.3), RColorBrewer (1.1-2), slingshot (2.2.0), TrajectoryUtils (1.2.0), princurve (2.1.6), scRNAseq (2.8.0), pathview (1.34.0), limma (3.50.0), dynamicTreeCut (1.63-1), dendextend (1.15.2), pheatmap (1.0.12), clustree (0.4.4), ggraph (2.0.5), msigdbr (7.4.1), kableExtra (1.3.4), scran (1.23.1), org.Mm.eg.db (3.14.0), AnnotationDbi (1.56.2), batchelor (1.10.0), dittoSeq (1.6.0), SingleR (1.8.1), scMerge (1.10.0), scater (1.22.0), scuttle (1.4.0), AnnotationHub (3.2.1), BiocFileCache (2.2.1), dbplyr (2.1.1), DropletUtils (1.14.2), RCurl (1.98-1.5), cowplot (1.1.1), scales (1.1.1), Matrix (1.4-0), forcats (0.5.1), stringr (1.4.0), dplyr (1.0.7), purrr (0.3.4), readr (2.1.2), tidyr (1.1.4), tibble (3.1.6), ggplot2 (3.3.5), tidyverse (1.3.1), SeuratObject (4.0.4), Seurat (4.1.0), SingleCellExperiment (1.16.0), SummarizedExperiment (1.24.0), GenomicRanges (1.46.1), GenomeInfoDb (1.30.1), IRanges (2.28.0), S4Vectors (0.32.3), MatrixGenerics (1.6.0), matrixStats (0.61.0), GEOquery (2.62.2), Biobase (2.54.0) and BiocGenerics (0.40.0).
scRNA-seq and analyses of 4T1 tumours for TGFβ communication patterns
We performed CITE-seq of dissociated 4T1 tumours. Tumours were digested as previously stated, sorted for viability and stained with TotalSeq-B Mouse Universal Cocktail, V1.0 (199902, BioLegend), TotalSeq-B0109 anti-mouse CD16/32 antibody (101345, BioLegend) and TotalSeq-B0301-5 anti-mouse Hashtag 3 (BioLegend), all according to the manufacturer’s instructions. Samples were then used as input into the Chromium 3′ single-cell kit for feature barcoding (PN-1000121, 10X Genomics). Libraries were prepared according to manufacturer’s instructions (CG000206, User Guide) and sequenced to 29,000 mean reads per cell for the gene expression library and 16,000 reads per cell for the multiplexing and feature barcoding libraries on a NS2000 P2 flow cell. Data were mapped using Cell Ranger v7.1.0 to the mouse genome (mm10-2020-A – 10X Genomics) in multi-mode. We analysed the data using R v4.3.3 (‘Angel Food Cake’). Quality control and annotation were performed as stated above. Cell identities were assigned using SingleR and the Immunological Genome Project cell-type reference. Then, we used the CellChat (v1.6.1) library to analyse the cell–cell communication patterns. We filtered out the cell–cell communication patterns involving fewer than 10 cells in the dataset. We then plotted the abundance of genes in the TGFβ signalling pathway and used the function netAnalysis_signalingRole_scatter to plot a scatterplot of the incoming–outgoing interaction strengths for the TGFβ signalling pathway.
scRNA-seq analyses of tumours from patients with breast cancer
To analyse the presence of neutrophils showing the Ly6CLow signature in patients with breast cancer, we used the dataset available with GEO accession number GSE114727. We performed quality control, and cells with mitochondrial transcripts above 10% were discarded (we used AnnotationHub to retrieve chromosomal localizations), as well as cells with a low number of detected features or low total counts (less than 10% of the mean). Then, counts were log normalized using the logNormCounts function, and datasets were merged using the scMerge library. Cell annotation was performed using the SingleR library and the HumanPrimaryCellAtlasData() function of the Celldex library. Batch correction was performed using the batchelor library, and then we kept only the neutrophils for subsequent analyses. High-variable gene selection was performed with the getTopHVGs function, keeping all genes above the fit for the variance of the log-normalized expression values for each gene across all cells in the population, calculated with the modelGeneVar function. PCA was then calculated using Scater and trimmed using the elbow calculation of the PCAtools library. tSNE was calculated using the Scater library. Clustering was done using k-means, and the number of centres was computed using the clusGap and maxSE functions of the cluster library. Cluster-specific genes were detected using the findMarkers function of the scran library, using the Wilcoxon rank-sum test, and keeping those with an FDR under 0.05. Category terms from these markers were calculated using the goana function from the limma library. Pseudotime analyses were conducted with slingshot, with no pre-determined starting cluster. The Ly6CLow neutrophil signature generated earlier in our mouse RNA-seq study (Fig. 4b and Supplementary Table 10) was then converted to human homologue genes using biomaRt, the getLDS function, and the mouse (mmusculus_gene_ensembl) and human (hsapiens_gene_ensembl) datasets. Then, the signature genes present in the dataset were used in the sumCountsAcrossFeatures function to aggregate the expression of the signature genes across cells and compare the expression of the Ly6CLow neutrophil signature in the different clusters. The session used the following libraries: cluster (2.1.2), celldex (1.4.0), data.table (1.14.2), enrichplot (1.14.1), ggpubr (0.4.0), GO.db (3.14.0), clusterProfiler (4.2.2), genesorteR (0.4.3), RColorBrewer (1.1-2), slingshot (2.2.0), TrajectoryUtils (1.2.0), princurve (2.1.6), scRNAseq (2.8.0), pathview (1.34.0), limma (3.50.0), dynamicTreeCut (1.63-1), dendextend (1.15.2), pheatmap (1.0.12), clustree (0.4.4), ggraph (2.0.5), msigdbr (7.4.1), kableExtra (1.3.4), scran (1.23.1), org.Hs.eg.db (3.14.0), AnnotationDbi (1.56.2), batchelor (1.10.0), dittoSeq (1.6.0), SingleR (1.8.1), scMerge (1.10.0), scater (1.22.0), scuttle (1.4.0), AnnotationHub (3.2.1), BiocFileCache (2.2.1), dbplyr (2.1.1), DropletUtils (1.14.2), RCurl (1.98-1.5), cowplot (1.1.1), scales (1.1.1), Matrix (1.4-0), forcats (0.5.1), stringr (1.4.0), dplyr (1.0.7), purrr (0.3.4), readr (2.1.2), tidyr (1.1.4), tibble (3.1.6), ggplot2 (3.3.5), tidyverse (1.3.1), SeuratObject (4.0.4), Seurat (4.1.0), SingleCellExperiment (1.16.0), SummarizedExperiment (1.24.0), GenomicRanges (1.46.1), GenomeInfoDb (1.30.1), IRanges (2.28.0), S4Vectors (0.32.3), MatrixGenerics (1.6.0), matrixStats (0.61.0), GEOquery (2.62.2), Biobase (2.54.0), BiocGenerics (0.40.0) and biomaRt (2.50.3).
Spatial transcriptomic analysis of 4T1 tumours
FFPE tissue blocks were cut down to approximately 6.5 mm × 6.5 mm in size and sectioned onto the capture areas of the Visium slide according to 10X Genomics Tissue Preparation Guide CG000408. Slides were then deparaffinized and H&E-stained according to the 10X Genomics Demonstrated Protocol CG000409 and imaged at the Animal & Tissue Imaging Core Facility at CSHL.
After imaging, the tissue was decrosslinked, and probe hybridization with the Visium Mouse Transcriptome Probe Kit (PN-1000365, 10X Genomics) was performed overnight at 50 °C. Probe ligation, probe release and extension, and library preparation were performed according to the manufacturer’s instructions, and final libraries were sequenced on a NextSeq 500 System (Illumina) to target 20,000 reads per spot. Sequencing data were processed with Space Ranger v2.0.0 (10x Genomics).
Further analyses were performed using R (4.2.0) and the Giotto library. In brief, the four sequenced tissues were combined in a Giotto object that was subsequently normalized using the normalizeGiotto function. We next calculated highly variable features; harmonized the datasets (using the runGiottoHarmony function); and further calculated the nearest network, Leiden clusters and spatial networks using Giotto. Unbiased clusters based on transcriptomic data were then assigned to necrotic, perinecrotic or non-necrotic regions of the tissue based on visual inspection of the clusters and the H&E staining. Cluster markers were detected using the ‘scran’ method of the findMarkers_one_vs_all function from Giotto, and Gene Ontology term analyses were performed using gprofiler2 using the cluster markers detected by the findScranMarkers Giotto function, combining all perinecrotic clusters and comparing them with all non-necrotic clusters. Fold change was subsequently used to define the upregulated and downregulated genes, which were subjected to Gene Ontology term analysis using gprofiler2. For the resolution enhancement used in the plots of individual genes in Fig. 3e and Extended Data Fig. 5k, we used BayesSpace. To compute spatial pathway enrichment, we used GOfuncR to query the genes in particular Gene Ontology terms and then used the Gene Ontology-associated genes for PAGE (parametric analysis of gene set enrichment) score. The session used the following packages: gprofiler2 (0.2.1), enhancedvolcano (1.14.0), ggrepel (0.9.1), ggpubr (0.4.0), ggplot2 (3.3.6), BayesSpace (1.6.0), SingleCellExperiment (1.18.0), GOfuncR (1.16.0) and Giotto (2.0.0.998).
Spatial transcriptomic analysis of NSCLC
To analyse the spatial relationship between neutrophils and cancer cells using spatial transcriptomics, we analysed a public domain NSCLC26 dataset using the provided Giotto object in R (v4.2.0 ‘vigorous calisthenics’). We first normalized the dataset using the normalizeGiotto function (with log normalization, library size normalization, and feature and cell scaling). We next calculated highly variable features, the nearest network, Leiden clusters and spatial networks using Giotto, and we identified genes with spatially coherent expression profiles using binSpect (method set as k-means). We then found the genes associated with the proximity to other cell types using the findICF() function (with the scaled values and Delaunay network, clustered by cell type and using the Wilcoxon rank-sum test, with a minimum of 20 unique and interacting cells, and FDR as the adjustment method, for 100 permutations). From the list, we selected the interaction changing genes of tumour cells interacting with neutrophils and used that list to analyse the associated Gene Ontology terms with the goana library. Finally, we plotted the results using ggplot2. The session used the following libraries: limma (3.52.0), scran (1.24.0), scuttle (1.6.0), SingleCellExperiment (1.18.0), SummarizedExperiment (1.26.1), GenomicRanges (1.48.0), GenomeInfoDb (1.32.1), MatrixGenerics (1.8.0), matrixStats (0.62.0), org.Hs.eg.db (3.15.0), AnnotationDbi (1.58.0), IRanges (2.30.0), S4Vectors (0.34.0), Biobase (2.56.0), BiocGenerics (0.42.0), ggplot2 (3.3.6) and Giotto (2.0.0.9021).
Human samples
We identified patients (female, mean of 56 years of age) with newly diagnosed stage I–III TNBC from electronic medical records in the Northwell Health system. We excluded patients with active or a previous history of malignancies (except for treated localized skin malignancies); those who had previously had any systemic therapies, including chemotherapy, hormonal therapy, molecular-targeted therapy and/or immunotherapies, for any reason before sample collection through core needle biopsy; those who had an active infection, uncontrolled rheumatological problems or known deep vein thrombosis or pulmonary embolism at the time of biopsy; and those who were or had been on systemic steroids and/or non-steroidal anti-inflammatory drugs within 30 days before biopsy. Archival FFPE tissue samples were identified by a breast pathologist at Northwell Health. This study was approved by the Institutional Review Board (protocol number: 21-0064) at Feinstein Institutes for Medical Research Northwell Health, and a waiver of informed consent was granted based on the retrospective nature of the study.
Analysis of MRI data from tumour necrosis in patients with breast cancer
Patient imaging and metadata were taken from the Duke-Breast-Cancer-MRI8 dataset12 deposited in the Cancer Imaging Archive. We included the first 200 patients in the dataset in the analysis. Recurrence-free survival, overall survival and tumour-type data were extracted from the provided clinical data sheets. Images from dynamic contrast-enhanced bilateral MRI studies were independently analysed for enhancement patterns by two independent investigators, including one breast radiation oncologist, who were blinded to the outcome data. Patients were categorized into those with primary tumours containing necrotic areas (defined as tumours with internal regions of low-contrast-enhanced signal) and those with primary tumours not containing necrotic areas (defined as tumours with homogeneous, high-contrast-enhanced signal). Radiological evidence of axillary or internal mammary adenopathy was noted during image review.
Statistical analysis
Unless otherwise indicated, data are represented as mean + s.e.m. Paired or unpaired, two-tailed t-tests or Wilcoxon rank-sum test were used to compare two groups, and more than two datasets were compared using one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test or Kruskal–Wallis test. Where applicable, normality was estimated using D’Agostino and Pearson or Shapiro–Wilk normality tests. log-rank (Mantel–Cox) analysis was used for Kaplan–Meier survival curves. The tests used are stated in the figure legends. No samples were excluded. All statistical analyses, except for RNA-seq analyses (see the section ‘RNA-seq’), were performed using Prism v8, v9 or v10 (GraphPad). A P < 0.05 was considered statistically significant; non-significant (NS) differences are indicated in the figures.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.