Plant materials and growth conditions
Col-0 was used as the wild type unless stated otherwise. ein2-1, etr1-3, ate1-2;ate2-1, prt6-5, proWOX4-erYFP, proPXY:GUS, proPXY:erVenus and 35S:EIN3-GFP have been described previously10,13,27,37,38,39,40. ate1-2;ate2-1 mutants were reciprocally backcrossed into Col-0 for three generations and transfer DNA insertions were confirmed by genotyping to obtain double-homozygous mutants.
Seeds were sown on half-strength MS (Duchefa) plates supplemented with 0.05% MES (Duchefa), 1% agar (Duchefa) and 1% sucrose (Duchefa), pH 5.8. MS medium also contained vitamins for all experiments except for ethylene measurement from roots. After incubation at 4 °C for more than 2 days, the plates were moved to the growth chamber (22 °C; 16 h light, 8 h dark). The day that the plates were placed in the growth chamber is defined as day 0. The seedlings were moved to the new MS agar plate around day 7; for the experiment in shoots, the seedlings were transferred to soil 1 or 2 weeks after germination and were grown in a greenhouse (16 h light, 8 h dark) unless stated otherwise. For examining normal periderm, we used 14- or 16-day-old seedlings as periderm is established at that age. For assessing regeneration, we usually used 17- to 21-day-old seedlings.
The Arabidopsis Genome Initiative locus codes for the genes are as follows: ATE1, AT5G05700; ATE2, AT3G11240; EBF1, AT2G25490; EIN2, AT5G03280; EIN3, AT3G20770; ETR1, AT1G66340; PBP1, AT3G16420; PCO1, AT5G15120; PCO2, AT5G39890; PER15, AT2G18150; PER49, AT4G36430; PRT6, AT5G02310; PXY, AT5G61480; RPS5A, AT3G11940; WOX4, AT1G46480.
Cloning
From the Col-0 genome, the 643-base-pair (bp) EBF1 3′ UTR with its downstream 72-bp sequence (from +2780 to +3495) was amplified by PCR using H122_EBF1_URT_F and H123_EBF1_URT_R primers18 (Supplementary Table 1), which have a 25-bp overlap with the erVenus and attL2 sequence, respectively (EBF1 3′ UTR). erVenus/pDONR plasmid (30 ng) was amplified with the EBF1 3′ UTR PCR product (600 ng) by PCR. The amplified PCR product was digested with DpnI enzyme at 37 °C for 2 h and was used for transformation into Escherichia coli (DH5α) by electroporation (erVenus–EBF1 3′ UTR/pDONR). RPS5A:erVenus–3AT/VD8034GW-mTurq and RPS5A:erVenus–EBF1UTR–3AT/FRm43GW were generated from RPS5A/pDONR, 3AT/pDONR, FRm43GW, VD8034GW-mTurq and erVenus/pDONR or erVenus–EBF1 3′ UTR/pDONR, respectively, by MultiSite Gateway LR clonase reaction.
From the Col-0 genome, promoter sequences of 1.1 kilobases (kb) of PCO1 (from −958 to +198) and 2.5 kb of PCO2 (from −2213 to +298) were amplified by PCR using H68_proPCO1_F and H69_proPCO1_R, or H70_proPCO2_F and H71_proPCO2_R primers, respectively23 (Supplementary Table 1). The amplified PCR products were cloned into the p1R4z-pDONR vector by BP reaction (proPCO1/p1R4z-pDONR, proPCO2/p1R4z-pDONR). proPCO1:erVenus–3AT/FRm43GW and proPCO2:erVenus–3AT/FRm43GW were generated from erVenus/pDONR, 3AT/pDONR41, FRm43GW42, and proPCO1/p1R4z-pDONR or proPCO2/p1R4z-pDONR, respectively, by MultiSite Gateway LR clonase reaction.
The plasmids generated in this work were introduced into Col-0.
Surgical injury of the periderm and chemical treatment
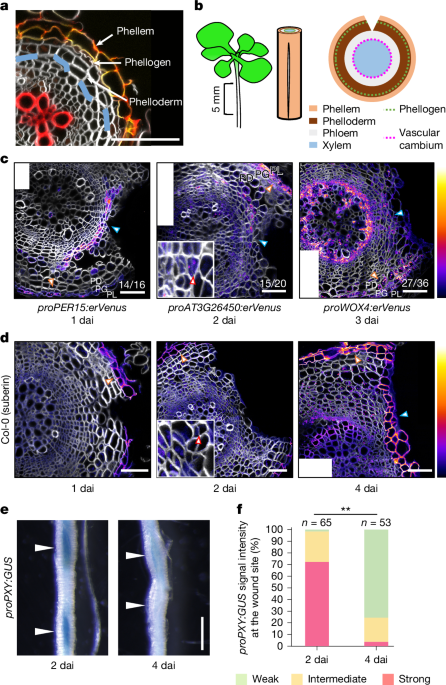
Roots within 5 mm below the root–hypocotyl junction were used for the wounding experiment unless stated otherwise. Under a dissection microscope, the shoot was pulled upwards slightly to create tension in the roots; the roots were longitudinally cut with a razor blade. As roots where the cut reached the vascular cambium region tended to form a callus-like structure instead of the wound periderm at 4 dai, we focused on sections in which the depth of the cut reached between the phloem parenchyma contacting the periderm to the phloem-side cambium in the following analysis unless stated otherwise. For the analysis of promoter induction at the wound site, we also excluded the sections in which the cut was just at the primary phloem pole because reporter expression tended not to be induced. To peel off the periderm for the oxygen level measurement, we made a shallow cut on the surface of the roots tangentially; the cut edge at the wound site was grasped with forceps and pulled towards the root tip.
For chemical treatment, 100 mM ACC (Merck) in water, 100 mM abscisic acid (ABA; Duchefa) in ethanol, 100 mM jasmonic acid (JA; Sigma-Aldrich) in dimethylsulfoxide, 100 mM AVG (Sigma-Aldrich) in water and 100 mM AgNO3 (Sigma-Aldrich) in water were prepared as stock solutions. Right after the injury, seedlings were moved to plates supplemented with 10 µM ACC, 10 µM JA, 10 µM ABA, 10 µM AVG or 0.5 mM AgNO3. For ACC treatment and its mock control, the plates were sealed with surgical tape and Parafilm. In all other experiments, plates were sealed only with surgical tape. For ABA and JA treatment, the MS agar plates containing an equivalent volume of ethanol or DMSO were used as the controls. For ethylene treatment, plates were placed in a 2-l container and 600 µl of 10% ethylene gas was injected (final concentration: 30 ppm). To ensure a consistent ethylene concentration, the container was opened and ethylene was re-injected every 1 or 2 days.
For the wounding experiment in shoot, we used an inflorescence stem whose length was between 10 and 15 cm. The region 1 to 2 cm below an inflorescence meristem was used for the surgery; we longitudinally cut the stem with a razor blade under a dissection microscope. For ACC treatment in shoot, seedlings were grown on MS agar plates for around 19 days. Inflorescence stems approximately 5 mm in length were used and a region of 2 to 3 mm below the inflorescence meristem was longitudinally injured. After the injury, seedlings were transferred to MS agar plates supplemented with or without 10 µM ACC. The plates were sealed with surgical tape and Parafilm.
To seal the wound site, lanolin was mixed with the equivalent volume of milliQ water. We applied lanolin or Vaseline to the wound after the injury. We also applied lanolin or Vaseline at the wound of control samples just before sampling to ensure that lanolin and Vaseline equally affected the histological processes.
For submergence treatment, the seedlings were first submerged into MS liquid medium not supplemented with sucrose, and their roots were injured. The seedlings were placed in the growth chamber for 24 h.
For oxygen treatment with reporter lines, MS liquid medium without sucrose was aerated with ambient air or oxygen gas for more than 10 min at 20 to 22 °C. Immediately before the surgical injury, the seedlings were submerged into aerated or oxygenated MS liquid medium and their roots were injured with a razor blade. The seedlings were transferred to glass medium bottles filled with aerated or oxygenated MS liquid medium and were placed in the growth chamber for 24 h. The method for oxygen treatment with wild-type and mutants was the same, with the exceptions that the seedlings were not submerged into MS liquid medium before the injury and they were placed in the growth chamber for 4 dai.
For hypoxia treatment, plants were grown under 5% oxygen conditions using a Whitley H85 hypoxystation (Don Whitley Scientific). Seedlings were treated for 24 h.
Oxygen measurement
Oxygen measurement was performed using O2 microsensors as previously described with modifications20. We used 24- or 25-day-old wild-type roots for the measurement. Shoot and roots below the region for the measurement were fixed on a metal mesh with plastic tape and rubber bands, and the metal mesh was set in a chamber. After we inserted an O2 microsensor (Unisense A/S) into the region 8 to 10 mm below the root–hypocotyl junction at the depth of 70 µm from the surface, the chamber was filled with aerated deionized water. Quasi-steady-state of oxygen concentration was measured under light (50–60 µeinstein) and dark conditions; we defined the quasi-steady state as a state in which a change in oxygen concentration was less than 5% of the total concentration for at least 5 min. The microsensor was retracted from the root, and the aerated water was drained. To measure oxygen levels when periderm integrity was compromised, the metal mesh was retrieved from the chamber, and the surface of roots used for the intact root measurement was peeled off as described above. The metal mesh was again set in the chamber and the microsensor was inserted at the depth of 50 µm in the surface-removed region. The measurement was performed in the same way as described above. Using the same roots for the measurement, the respiration and photosynthetic rate should be almost identical, and the oxygen intrusion rate would be the main cause of the oxygen level changes before and after the surgery. After the measurement, the roots were retrieved to perform histological analysis.
Ethylene measurement
We injured roots within 3 to 4 cm below the root–hypocotyl junction in 24-, 25- or 26-day-old seedlings and removed their shoot by cutting off the hypocotyl. The hypocotyl cut sites were sealed with Vaseline. Immediately, roots were moved into a vial (volume 37.5 ml) that had been partially filled with approximately 25 ml 1% agar in 1× PBS. Plants were kept in the growth chamber (22 °C; 16 h light, 8 h dark), and samples were taken at the indicated times. After detecting the level of ethylene, we measured the fresh weight of roots and the headspace of the vial. The used Vaseline weight was subtracted from the measured fresh weight, and the corrected weight was used for quantification. Around 20 seedlings were used for measurement. For inflorescence stems, regions within 10 to 15 cm below the inflorescence meristem were used for the measurement; 3- to 5-cm stems were collected, and the flower stalks were cut. The fresh weight was measured, and the top, bottom and the flower stalk cut sites were sealed with Vaseline. For wounding, the stems were cut in the longitudinal direction with a razor blade. Immediately, the inflorescence stems were placed into a vial partially filled with approximately 25 ml 1% agar in 1x PBS. Around 30 inflorescence stems were used for measurement. In both cases, to measure the amount of ethylene emitted, 1-ml samples from the headspace were collected using a syringe 3, 6 and 24 h after the vial was closed. Ethylene measurements were performed using a Clarus 480 gas chromatograph (PerkinElmer) carrying a HayeSep N (80–100 MESH) 584 column. The oven temperature was 100 °C, with the flame ionization detector temperature set to 150 °C. The flow rate of the nitrogen carrier gas was 20 ml min−1. Peak area was integrated and compared against a standard curve.
GUS staining and microtome sectioning
Roots from 0 to 3 cm below the root–hypocotyl junction with a wound were submerged in 90% acetone on ice for 30 min. The samples were washed with 50 mM sodium phosphate buffer twice and were submerged in the GUS staining solution under vacuum for 30 to 60 min. The samples were incubated with GUS staining solution (30 mM Na2HPO4, 20 mM NaH2PO4, 1.5 mM K4Fe, 1.5 mM K3Fe, 500 mg l−1 X-Gluc, 0.1% Triton) at 37 °C until sufficient GUS signals were detected. The roots were fixed in fixation solution (50 mM sodium phosphate (pH 7.4), 4% formaldehyde, 1% glutaraldehyde) at 4 °C overnight. To observe the samples from a lateral view under a dissection microscope, we kept the samples in 70% ethanol to remove chlorophyll.
Vibratome sectioning and staining methods
The samples were fixed in 4% paraformaldehyde in 1× PBS for 30–60 min and washed with 1× PBS twice. The samples were embedded in 4% agarose in 1× PBS and 200 µm-thick cross-sections were made using a vibratome. The cross-sections were stained in 1× PBS or ClearSee43 supplemented with 1 µl ml−1 Renaissance SCRI 2200 (SR2200; Renaissance Chemicals) to stain cell walls44. To stain lignin, the cross-sections were stained in ClearSee supplemented with 1 µl ml−1 SR2200 and 50 µg ml−1 Basic Fuchsin (Sigma-Aldrich). For suberin staining, the cross-sections were stained in ethanol supplemented with 0.01% Fluorol Yellow 088. Before observation, the cross-sections were washed with 1× PBS supplemented with 1 µl ml−1 SR2200. To visualize lignin and suberin at the same time, first the cross-sections were stained in ClearSee supplemented with 1 µl ml−1 SR2200 and 50 µg ml−1 Basic Fuchsin overnight. Next, suberin staining was performed as described above, and before observation, the cross-sections were washed with 1×PBS supplemented with 1 µl ml−1 SR2200. To observe the root tip, the samples were fixed in 4% paraformaldehyde in 1× PBS for 30 to 60 min and washed with 1× PBS twice. The samples were then stored in ClearSee supplemented with 1 µl ml−1 SR2200.
Microscopy and data analysis
A Leica 2500 microscope (Leica) was used for light microscopy images and Leica SP5 (Leica), Stellaris (Leica) and LSM880 (Zeiss) confocal laser scanning microscopes were used to detect GFP, YFP, Venus, Basic Fuchsin, Fluorol Yellow 088 and SR2200.
Confocal images were stitched by using an ImageJ plugin (Pairwise stitching)45. Occasionally, this resulted in the formation of empty corners in the figure panels. To visually separate the empty corners from the black background signal of the microscopy image, we filled these empty corners with white colour.
Fluorescence signal intensities at the wound site were quantified using Fiji (v1.53) and PlantSeg. We defined the wound site as a vascular tissue located within 30 µm from the wound surface for roots. For quantification in roots, the cell wall images of the wound site were extracted by using Fiji, and the extracted images were used for segmentation by PlantSeg46. After manual modification, the segmented images were used to count the cell number at the wound site and to measure the Venus–YFP signal intensity in each cell by Fiji. The Venus signal intensities were measured with the minimum signal threshold to exclude the background signals. The Venus-positive cells were defined by whether the signal intensity was above the threshold specific to each line. Five to six roots were used for each treatment in each experiment; a maximum of two cross-sections of the same root at different positions were used for quantification. Cross-sections with cuts reaching the vascular cambium were excluded from quantification because they tended to show unstable periderm gene induction. For Venus signal intensity quantification in intact proPER15:erVenus and proPBP1:erVenus roots in Extended Data Fig. 2f, signal intensities were measured in periderm from the quarter of the cross-section with the minimum signal threshold. For quantification of periderm reporter lines in Extended Data Fig. 1c, five distal phloem cells in the intact tissues or near the wound in each cross-section were used for Venus signal intensity measurement with the minimum signal threshold. For RPS5A:erVenus and RPS5A:erVenus-EBF1UTR quantification in Extended Data Figs. 3h,i, 5a,b and 8e, five to ten distal phloem parenchyma cells in each cross-section were used for Venus signal intensity measurement with the minimum signal threshold. At 8 and 11 h after injury or at 2 and 5 dai, distal phloem parenchyma cells near the wound were selected for signal measurement. The average of Venus signal intensities from five to ten cells was used for statistical analysis. For proPXY:erVenus quantification in Extended Data Fig. 1e, we circled the vascular cambium and xylem parenchyma region and measured Venus signal intensities within the circle with the minimum signal threshold to exclude the background signals. The signal intensities were normalized with those of uninjured roots in each repeat. For proPCO1:erVenus and proPCO2:erVenus signal quantification in Fig. 3b and Extended Data Figs. 6b,c and 8f, the vascular cambium, xylem parenchyma and phloem parenchyma region were circled, and we measured Venus signal intensities within the circle with the minimum signal threshold to exclude the background signals. For RPS5A:erVenus and RPS5A:erVenus-EBF1UTR quantification in Extended Data Fig. 3j,k, Venus signal intensities were measured with the minimum signal threshold and averaged from five epidermal cells in each roots. For RPS5A:erVenus and RPS5A:erVenus-EBF1UTR quantification in Fig. 4h and Extended Data Fig. 10h,i, 10 cortical cells or 15 cortical cells near the wound in each cross-section were, respectively, used for Venus signal intensity measurement with the minimum signal threshold. For Extended Data Fig. 10i, the signal intensities were normalized with those of control inflorescence stems in each repeat. T2 or T3 lines were used. For proPER15:erVenus quantification in shoots, the wound site within 40 µm from the wound surface was selected and the Venus signal intensities were measured with the minimum signal threshold in each cross-section. For quantification of suberized cell formation in inflorescence stems in Extended Data Fig. 10j, the length of the total wound site and the region covered with suberized cells were measured and the ratio of the suberized region was calculated.
proPXY:GUS signals at the wound site were classified into three categories under a stereo microscope: weak when there were no or faint signals, strong when the signals were close to saturation, or intermediate when the signals were clearly visible and weaker than the strong category.
The density of suberized cells at the wound site was calculated by dividing suberized cell number by the length of the wound site using Fiji. For the quantification, the area connected to the original periderm was excluded because it was occasionally hard to distinguish which suberized cells were originated from vascular tissue. The cross-sections showing callus-like structure formation were also excluded as suberized cell formation and morphology at the wound site is affected by callus-like structure. The mature suberized cells (more than 20 µm2) were counted. For Extended Data Fig. 5e, when the re-established periderm had three gaps in the suberized cell layer or three successive cell files did not show suberized cells, we defined it as a failure in suberized cell layer formation.
Data analysis was performed with MS Excel v2308, R (v2024.04.2) and Python 3.9.
Statistics and reproducibility
In the box plots, the 25th, 50th (central value) and 75th percentile are marked with horizontal lines within the box. The ends of the whiskers indicate the maximum and minimum values within 1.5× the interquartile range from the box ends. Outliers are shown above or below the whiskers. For quantification of suberized cell formation and gene induction at the wound site or gene expression level in the vascular region, each dot corresponds to a cross-section. For ethylene concentration, each dot indicates a repeat. All experiments were repeated at least three times (three times for Figs. 1a,c–e, 2c,e, 3a,b and 4b,c,e and Extended Data Figs. 1a,b,d,f,g, 2a–d,l,m, 3a,b(AVG and AgNO3),c,d, 4a,b, 5a–d,f, 6a–c,h, 7b,c,g–i, 8a–d, 9a,c–h and 10a–c,e,g; four times for Figs. 2a,g, 3d and Fig. 4f and Extended Data Figs. 2g,h–j,n, 3b(ACC) and Extended Data Fig. 10d; five times for Fig. 4g and Extended Data Figs. 4c and 5e; six times for Extended Data Figs. 6f, 7a and 10f; nine times for Fig. 3f; all are biological repeats). Exact P values are provided in Supplementary Table 2.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.