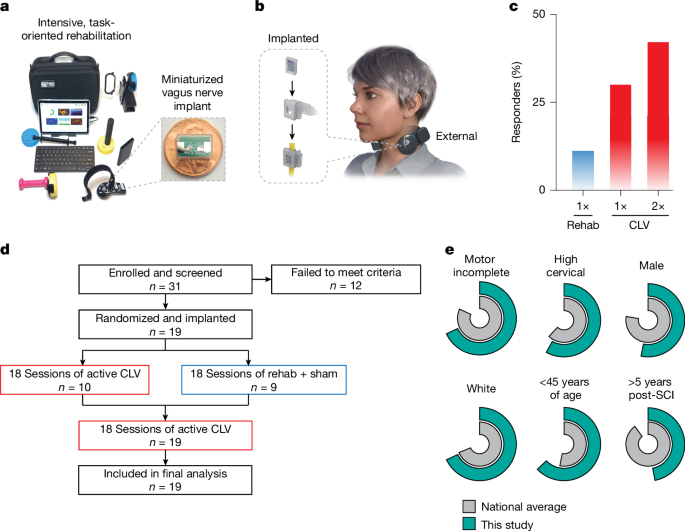
Overall study procedure
The trial was designed as a double-blinded, randomized, sham-controlled early feasibility study (Extended Data Fig. 1). The clinical protocol and informed consent document are included in the Supplementary Information. Individuals who indicated interest in participation and met general criteria in an initial screen underwent informed consent. After enrolment, all participants underwent implantation of the miniaturized VNS device. Approximately 4 weeks after implantation, all participants received a post-implantation baseline assessment. Participants then received 18 sessions of rehabilitation with active CLV or sham stimulation in accordance with their randomization. After the initial 18 sessions, participants received an additional 18 session of rehabilitation with active VNS, regardless of their previous group assignment. The primary objectives evaluated safety, feasibility and changes in measures of arm and hand function. Outcomes were assessed after enrolment, after implantation, after completion of 18 sessions of therapy and after completion of 36 sessions of therapy. Occurrence of adverse events was screened throughout the course of the study.
Regulatory compliance and ethics
The use of the miniaturized VNS device used in this study received investigational device exemption (approval from the FDA investigational device exemption approval ID G190032). In addition, all study procedures were approved by the Baylor Scott and White Research Institute Institutional Review Board, the University of Texas at Dallas Institutional Review Board, and the US Department of Defense Human Research Protections Office. The study was conducted in compliance with all relevant regulatory and ethical guidelines. The trial was preregistered at ClinicalTrials.gov (NCT04288245).
Participants
Participants were recruited from 5 March 2021 to 30 June 2023. Trial recruitment ended when budgeted funds for the trial were expended. Patient characteristics are delineated in Extended Data Table 1. A total of 293 potential participants contacted us or were identified through an established referral network at Baylor Scott and White Spinal Cord Injury Model System, study flyers, local advertisements and online advertisement. Of these, 19 met criteria, elected to participate, were consented and enrolled, and ultimately underwent implantation. Informed consent was obtained in a private setting after consultation with study staff to answer any questions. The informed consent process followed our established procedure, which is included in the Supplementary Information.
Participants met the following key inclusion criteria: (1) first time cervical SCI occurring at least 12 months before and resulting in AIS grade B, C or D (confirmed during the baseline visit by an experienced clinician); (2) residual movement in the upper limb and hand in either arm; (3) appropriate candidate for VNS implantation; (4) between 18 and 64 years of age; (5) a signed and dated informed consent form; and (6) willing to comply with all study procedures and were available for the duration of the study. Participants were excluded based on the following criteria: (1) SCIs by sharp objects, firearms, and non-traumatic or congenital causes; (2) evidence of recurrent laryngeal nerve injury; (3) excessive scar tissue in the neck; (4) concomitant clinically significant brain injuries; (5) previous injury to the vagus nerve; (6) previous or current treatment with VNS; (7) receiving any therapy that would interfere with VNS; (8) pregnant or lactating; (9) psychiatric disorders, psychosocial and/or cognitive impairment that would interfere with study participation; (10) abusive use of alcohol and/or illegal substances; (11) participation in other interventional clinical trials; (12) known immunodeficiency or receipt of chronic corticosteroids, immunosuppressants, immunostimulating agents or radiation therapy within 6 months; (13) significant comorbidities or conditions associated with high risk for surgical or anaesthetic survival; (14) active neoplastic disease; (15) significant local circulatory problems; (16) any medical condition or other circumstances that might interfere with their ability to receive rehabilitation or return for follow-up visits; (17) any condition that would preclude adequate evaluation of the safety and performance of the device; (18) aphasia and other cognitive deficits that interfere with provision of informed consent; (19) recent history of syncope; (20) recent history of dysphagia; (21) currently require, or are likely to require, diathermy; (22) significant respiratory issues that would interfere with participation; (23) non-English speaking; (24) acutely suicidal and/or have been admitted for a suicide attempt; and (25) incarceration or legal detention. Full eligibility criteria can be found on ClinicalTrials.gov (NCT04288245). No changes were made to eligibility criteria after trial commencement.
VNS device implantation
All 19 enrolled participants were implanted with a next-generation VNS device29. This device is over 50 times smaller than conventional VNS systems, largely due to the offloading of the battery and elimination of the need for leads. Both of these changes simplify surgical implantation by necessitating only a single incision at the neck and obviating the need for tunnelling. Implantation was performed by surgeons with experience in the implantation of VNS systems. After surgical preparation and induction of general anaesthesia, the skin and platysma overlying the left cervical vagus nerve were incised. The sternocleidomastoid muscle was mobilized laterally to reveal the carotid sheath, and the vagus nerve was dissected free circumferentially over a length of 3–4 cm. The vagus nerve was placed inside the silicone cuff, which positions the electrodes of the implanted pulse generator (IPG) in contact with the nerve. The silicone cuff was closed around the nerve with two 4-0 permanent sutures. The IPG was then positioned superficially to facilitate alignment with the external components during stimulation. Before closure of the skin, wireless communication and power functions of the IPG were verified. After confirmation of device functionality, closure of the platysma and skin with absorbable sutures was performed, followed by a second communication verification. Impedance checks confirmed that all participants were within the acceptable range. Average surgical time was 38 ± 2 min. Routine post-anaesthetic care was provided. Approximately 1 week later, participants returned to the clinic for a follow-up visit to assess recovery.
VNS delivery
Participants were randomized 1:1 to receive either 18 sessions of rehabilitation with sham stimulation followed by 18 sessions of rehabilitation with active VNS (n = 9) or 36 sessions of rehabilitation with active VNS (n = 10). A blocked software-randomized design was used with a block size of two. The blocking covariate was impairment severity (treated arm GRASSP ≤ 58 versus GRASSP > 58). Ten participants were randomized to receive active (0.8 mA, 30 Hz for 500 ms) VNS for all 36 sessions of VNS. Nine participants were randomized to receive sham VNS for the first 18 sessions and active VNS for the second 18 sessions (Extended Data Fig. 1).
Before the beginning of each rehabilitation session, vital signs were collected with a digital blood pressure cuff. During rehabilitation sessions, the external power and communication module (PCM) was placed in a band around the neck of the participant with the coil aligned over the IPG. Because stimulation was only delivered during rehabilitative training sessions, the PCM was only worn during these sessions. Each 0.5-s train of VNS was delivered concurrent with exercises during rehabilitative training (as detailed below), and comprised 0.8-mA 100-µs biphasic pulses at 30 Hz, as in previous studies.
The concurrent timing of VNS and movement is based on the principle that precisely timed neuromodulatory feedback can direct specific synaptic changes to enhance recovery. After an SCI, a subset of neurons within the central nervous system is affected by the injury, and promoting long-term potentiation and depression within these networks has long been recognized as an approach to support recovery1,50,51. The ability to direct synapse-specific, adaptive plasticity within the affected networks while not influencing other networks is at the core of the credit assignment problem, which articulates the challenge of identifying which synapses should be changed to improve motor function17,52,53,54. CLV leverages the synaptic eligibility trace, a phenomenon in which the arrival of neuromodulatory reinforcement within seconds promotes plasticity in recently active networks and not in inactive networks16,55,56,57, to direct changes within specific synapses. In CLV, motor networks controlling the upper limb affected by the SCI are engaged by rehabilitative exercises, and closed-loop VNS delivered in response to a target movement triggers neuromodulatory release to produce therapeutic synaptic plasticity within these networks3,11.
All participants underwent implantation, a key feature of blinding. In addition, all participants received equivalent individualized rehabilitation regimens and donned the external device during rehabilitation sessions, regardless of active or sham group assignment. The stimulation parameters were software controlled and were preset by a study staff member who was not involved in rehabilitation, data collection or analysis, allowing therapists and assessors to maintain blinding. Most participants did not perceive the stimulation or only felt the first few stimulations each day and rapidly adapted to the sensation. To maintain blinding, at each rehabilitation session, participants in the sham group received active stimulations of reducing strength beginning at 0.8 mA and decreasing with each trigger to 0.6 mA, then 0.4 mA, then 0.2 mA, then 0.1 mA and then 0 mA for the remainder of the session. Preclinical and clinical studies indicated that this amount of stimulation was insufficient for therapeutic effect and sufficient to ensure blinding. Participants were instructed that they may initially perceive stimulation, but the perception may fade. Participant blinding was confirmed with a questionnaire at the end of phase 1 of the study, in which 11 of 19 participants were incorrect or uncertain about group assignment. Although therapists and assessors were blinded, blinding status was not directly surveyed in these individuals and should be assessed in future studies to confirm effective concealment.
Personalized rehabilitation regimen
All 19 participants completed 36 sessions of intensive rehabilitation at a rate of approximately 3 per week. Each session was approximately 90 min long. Both groups followed the same visit schedule and received equivalent rehabilitation of one arm. Clinical judgement was used to select the arm that was most likely to benefit from therapy.
Personalized exercise regimens were selected based on the ability profile of each participant. Each regimen incorporated conventional rehabilitative exercises and training on a computer-based rehabilitation system28. As appropriate for their level of ability and expressed interest, participants also performed functional tasks (labelled as object manipulation in Fig. 2 and ‘Individualized therapy sessions’ in Supplementary Information), including jar opening, threading a nut on a bolt, lifting cans, writing, and inserting and turning keys. Participants generally cycled between relatively short (1–5 min) sets of each exercise to mitigate substantial fatigue. Difficulty was continuously monitored by licensed therapists to ensure that the therapeutic exercises were challenging. If performance improved, difficulty was increased by reducing the linear assistance factor (that is, gain) and increasing the game level of the computer-based rehabilitation system, which had the effect of increasing the required movement speed, accuracy and force28. Assistance factor adjustments to the exercise regimens were made systematically, such that a typical progression for a single exercise included decreasing the input multiplier from the controller by 20%. In addition, progression involved increasing the number of repetitions or extending the duration of the exercise.
The closed-loop triggering scheme to deliver VNS concurrent with movements used two strategies. In the first strategy, the therapist overseeing the rehabilitation session used a button press in a software app to trigger stimulation on movements identified as above-average attempts based on specific performance metrics, such as increased force, speed, accuracy or fluidity of motion. This is the stimulation approach that has been applied in conventional studies using paired VNS therapy43,58,59. In the second approach, triggering was automatically controlled by a software algorithm, as previously described30. This strategy used real-time movement signals collected from the sensors in the rehabilitative training devices and delivered stimulation on movements that exceeded a continuously updated threshold. The relevant aspect of the movement for triggering was dependent on the gameplay; for example, gameplay controlled by pinch force used the force signal to determine triggering, whereas gameplay controlled by wrist rotation used degree of rotation to determine triggering28,35. The algorithm initiated stimulation within 500 ms when movement exceeded the 95th percentile of previous repetitions. The algorithm produced stable triggering across a range of impairments and over the course of rehabilitative sessions (Extended Data Fig. 2). Most participants received a combination of both stimulation-triggering strategies.
Individuals with lived SCI experience, including study participants, were iteratively involved in the design of the CLV system and therapy, including the development and implementation of the PCM and neckband, the rehabilitation devices and the design of the rehabilitative regimens. The components were designed to emphasize adoption among individuals with SCI, maximize comfort and promote engagement with the therapy. Consistent with this, study participants indicated a high level of satisfaction with the therapy (4.6 ± 0.2 on a 5-point Likert scale surveyed at the end of the study), a critical consideration for eventual clinical adoption.
Outcome assessments
GRASSP
The GRASSP (version 1) is a clinician-administered assessment quantifying three domains describing upper limb function and impairment60. This assessment was specifically designed to evaluate the effect of novel interventions on upper limb impairment in the traumatic tetraplegic population. The preregistered clinical end point was a greater than four-point increase in GRASSP. An improvement of six points or more in the trained limb was considered a meaningful difference. Higher scores are associated with improved arm and hand function. GRASSP scores are categorical and were collected at each assessment session. Percent disability, as reported in the main text, was derived from GRASSP scores. This metric was calculated as the number of GRASSP points gained during therapy as a proportion of the difference between baseline pre-therapy score and the total number of available points to reach a maximum score.
Quantitative force and range of motion assessment
A suite of rehabilitative tools was used to assess force and range of motion in the hand and wrist throughout the course of the study28,35. The system includes modules to quantify wrist rotational range of motion and torque on a doorknob and a revolving D-handle, wrist flexion–extension range of motion and torque, and finger flexion–extension force. These continuous values were collected at each rehabilitation session, as appropriate for the assigned exercises for each participant. The preregistered strength end points were greater than 10% increases in finger pinch and flexion force following active VNS, 10% increases in wrist flexion and extension force following active VNS, and 10% increases in wrist pronation and supination force following active VNS.
Jebsen–Taylor Hand Function Test
The Jebsen Taylor Hand Function Test is a widely used standardized and objective measure of fine and gross motor hand functions that uses simulated activities of daily living61. This was an exploratory end point.
Spinal cord independence measure
As this study only performed hand and arm rehabilitation, we did not expect to see improvements in respiration, lower body function, bowel function, bladder function or mobility. We thus excluded these metrics from SCIM-III to produce an 18-point measure of independence associated with arm and hand function. This composite score includes feeding, grooming, toileting, upper body bathing and upper body dressing, all of which require arm and hand function. This was an exploratory end point.
American Spinal Injury Association Impairment Scale
The AIS is the gold-standard assessment to evaluate level and completeness of injury in individuals with SCI. In this study, AIS was evaluated by R.G.H., a clinician with extensive expertise in SCI, at baseline and at the end of each phase. AIS grade was used to confirm study eligibility.
Data analysis
The primary and secondary study outcomes were reviewed by the regulatory bodies and were preregistered on ClinicalTrials.gov (NCT04288245). Data in the figures and text are presented as mean ± standard error of the mean unless otherwise indicated. Comparisons across groups were made using unpaired Student’s t-tests or Wilcoxon rank-sum tests. As appropriate, comparisons across time were made using paired Student’s t-tests or Wilcoxon signed-rank tests. Before conducting any parametric test, the assumption of normality was first checked using the Jarque–Bera goodness-of-fit test. For Fig. 4 and Extended Data Fig. 3, a linear mixed model was fitted to the data from each task, and statistics were performed using that model. Pearson correlation tests were used to determine the correlation between a number of elements and GRASSP score changes. Standardized effect size was calculated using Cohen’s d. As there was high test–retest reliability (Pearson correlation coefficient, R = 0.95, P < 0.00001) for the baseline assessments performed before and after implantation and no significant difference (P > 0.05), these two values were averaged to serve as the baseline assessment. All comparisons used an α = 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.