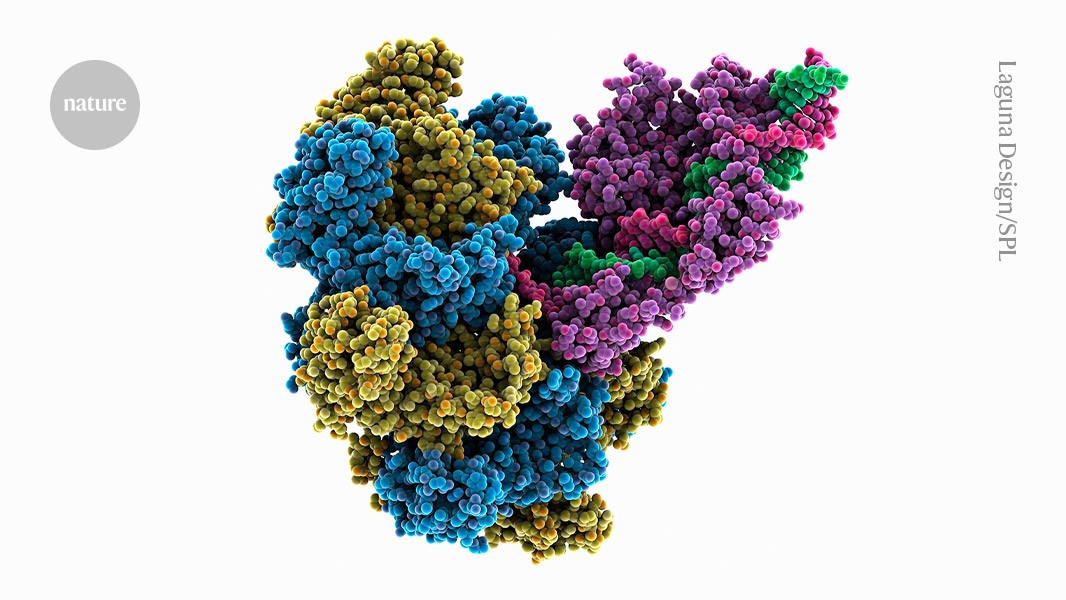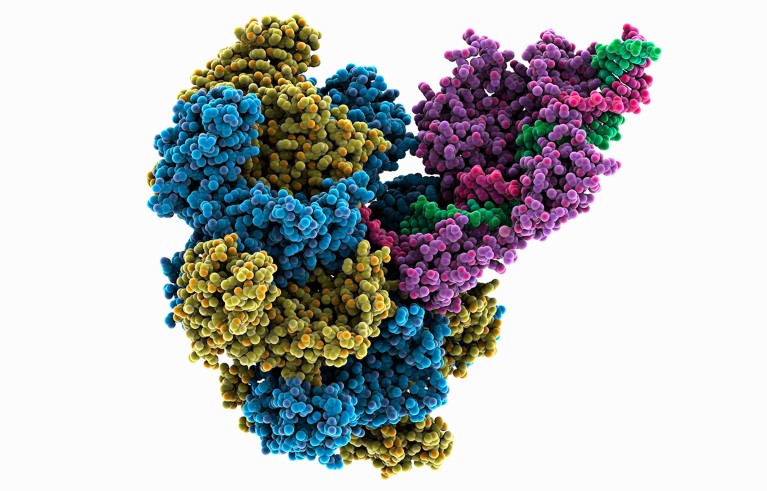
A system that can add an entire gene to the genome uses enzymes called transposases (yellow, blue, purple; artist’s impression) to alter DNA (red, green). Credit: Laguna Design/Science Photo Library
An innovative genome-editing tool promises to do what original CRISPR systems have struggled to achieve: insert entire genes, precisely and efficiently, into human DNA.
Described today in Science1, the method could pave the way for gene-correction therapies that would be given once, and work regardless of the specific mutation causing an individual’s disease. It could also accelerate the development of engineered cell therapies for cancer and simplify the creation of genetic models for research.
“It could really be a big part of the future,” says study co-author David Liu, a chemical biologist at the Broad Institute in Cambridge, Massachusetts.
Special delivery
One of the most commonly used gene-delivery methods relies on engineered viruses to insert stretches of genetic material into a cell’s genome. Although effective, these viruses tend to insert their payloads at random, risking harmful disruptions or poor control over gene expression.
CRISPR offers more control than viral carriers do, but typically requires the cutting of DNA — raising the chances of unwanted mutations and incomplete repair — or the designing of custom templates for each mutation, limiting its scalability.
UK first to approve CRISPR treatment for diseases: what you need to know
The new system circumvents both problems by introducing full-length genes at targeted sites in a single step, without cutting DNA or needing bespoke designs. Developed by Liu, biochemist Samuel Sternberg at Columbia University in New York City and their colleagues, the method uses a bacterial enzyme complex called a CRISPR-associated transposase, or CAST.
Transposases are enzymes that power the movement of ‘jumping genes’ — ‘selfish’ bits of DNA that hop around the genome to propagate themselves. Researchers have already repurposed CAST systems to shuffle large chunks of genetic material in bacterial cells. But in human and other mammalian cells, all previously reported versions of CAST have struggled with low efficiency.
Evolving enzymes
To overcome these barriers, Liu and Sternberg turned to directed evolution, a technique that harnesses the power of Darwinian selection in the laboratory. They put the key genes encoding the components of a CAST system into a bacteriophage, a virus that infects bacteria. Their set-up ensured that such viruses with the most effective versions of CAST — those that integrated DNA into the genome quickly and accurately — would grow best.
Then, they let evolution run its course.
After hundreds of viral generations, and rational engineering of some CAST components, the researchers produced an optimized version of the enzyme complex. This had 21 small changes across five proteins that contribute to the CAST architecture — an achievement that pushes the limits of protein design, notes Makoto Saito, a bioengineer at RIKEN in Wakō, Japan. “This is crazy directed evolution!” he says.
CRISPR cancer trial success paves the way for personalized treatments
The resulting complex, termed evoCAST, demonstrated an insertion efficiency of up to 30% across multiple genomic sites — a more than 400-fold improvement over the non-evolved original.
In laboratory tests, evoCAST successfully integrated segments of more than 10,000 nucleotides, long enough to deliver entire genes and their control elements. It worked across a variety of human cell types, targeting genomic “safe harbour” sites that can accommodate new DNA without disrupting cellular functions and installing genetic payloads at the natural locations of multiple disease-related genes.
Notably, evoCAST delivers its genetic cargo in a single enzymatic step, without creating double-stranded breaks in the genome.