Generation of experimental mice
The Slc6a6 mice were bred as described previously25. For all mouse leukaemia experiments, male and female Slc6a6+/+ and Slc6a6−/− mice were used as donors and B6-CD45.1 (B6.SJL-PtprcaPepcb/BoyJ) or C57BL6/J mice were used as transplant recipients. For xenograft experiments with human cells, NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) were used as transplant recipients. All mice were 6–16 weeks of age. All animals used for in vivo studies were randomly selected to receive either control or treatments. Animals were selected based on genotype, age and sex. No statistical method was used to predetermine sample size for experiments. Adequate sample size was determined based on previous publications1,3,6. Mice were bred and maintained in the animal care facilities at the University of Rochester. All animal experiments were performed according to protocols approved by the University of Rochester’s Committee on Animal Resources. Mice were housed with the same sex in ventilated cages, under a 12 h–12 h light–dark cycle with temperature (64–79 °F) and humidity (winter levels, <30%; summer levels, >70%) control. Mice were given enrichment material and fed a standard chow diet. Irradiated recipients were maintained on acid water HYDROPAC (pH 2.5–3.0) and irradiated sulfatrim diet (Mod LabDiet 5P00 with 0.025% trimeth and 0.124% sulfameth). NSG recipients were housed in BSL-2 facility. Cdo1fl/fl mice were generated in collaboration with the Transgenic and Genome Editing Core Facility at Augusta University using CRISPR–Cas9 gene editing. These mice were bred to the MSC-specific Prrx1-cre (B6.Cg-Tg(Prrx1-cre)1Cjt/J) to generate a Cdo1fl/flPrrx1-cre+ mice, where taurine production is blocked in the MSC/osteolineage cells. As Prrx1-Cre can be expressed in the female germline, only male Prrx1-cre+ mice were used for breeding and experiments. Blinding was not relevant to mouse experiments as researchers needed to know the conditions for each experiment. Flow cytometry, FACS, western blots, Seahorse analysis, sequencing and imaging used analytical machines and blinding was therefore not necessary.
Cell isolation and FACS analysis
Cells were suspended in Hanks’ balanced salt solution (Gibco) with 5% FBS and 2 mM EDTA. Cells were prepared for FACS analysis and sorting as previously described1,6. Antibodies used for defining haematopoietic cell populations were as follows: CD3ε, CD4, CD8, GR1, CD11b, TER119, CD45R and CD19 (all for lineage), KIT, SCA1, CD48 and CD150. All antibodies were purchased from, eBioscience, BioLegend or BD Biosciences. A detailed list of antibodies is provided in Supplementary Table 2. Analysis was performed on LSRFortessa (BD Biosciences), and cell sorting was performed on the FACSAria II (BD Biosciences). Data were analysed using FlowJo.
Retroviral and lentiviral constructs and virus production
Retroviral MSCV-BCR-ABL-IRES-GFP (or -tNGFR) and MSCV-NUP98-HOXA9-IRES-YFP (or -huCD2 and -tNGFR) were used to generate bcCML. AML was generated with MSCV-MLL-AF9-IRES-tNGFR and MSCV-NRAS(G12V)-IRES-huCD2 or MSCV-AML-ETO9a and MSCV-NRAS(G12V)-IRES-YFP. RAGA (Q66L; Addgene, 99712) and RHEB (Q64L; Addgene, 64607) were cloned into the MSCV-mCherry backbone (Addgene, 52114). Lentiviral shRNA constructs were designed and cloned into the pLV-hU6-EF1a-green or pLV-hU6-EF1a-red backbone (Biosettia) according to the manufacturer’s protocol. A detailed list of shRNA sequences is provided in Supplementary Table 1. Virus was produced in 293T cells (ATCC) transfected with viral constructs along with VSV-G, Gag-Pol (retroviral production) or pRSV-rev, phCMV and pMDlg/pRRE (lentivirus production) using X-tremeGENE-HP reagent (Roche). Viral supernatants were collected for 3 to 6 days followed by ultracentrifugal concentration at 20,000 rpm for 2 h.
Generation and analysis of leukaemia models
Bone marrow KLS cells were sorted from Slc6a6+/+ or Slc6a6−/− mice and cultured overnight in X-VIVO15 (Lonza) medium supplemented with 10% FBS (GeminiBio), 50 μM 2-mercatpoethanol, SCF (100 ng ml−1, R&D Systems), TPO (10 ng ml−1, R&D Systems) and penicillin–streptomycin (Gibco). Cells were retrovirally infected with MSCV-BCR-ABL-IRES-GFP (or -tNGFR) and MSCV-NUP98-HOXA9-IRES-YFP (or -huCD2 or -tNGFR) to generate bcCML. AML was generated by sorting bone marrow KLS cells from Slc6a6+/+ or Slc6a6−/− mice and culturing in RPMI medium (Gibco) supplemented with 20% FBS, 50 μM 2-mercaptoethanol, 100 ng ml−1 SCF (R&D Systems), 10 ng ml−1 IL-3, and 10 ng ml−1 IL-6 (R&D Systems). Cells were retrovirally infected with MSCV-MLL-AF9-IRES-tNGFR and MSCV-NRAS(G12V)-IRES-YFP cells were collected 48 h after infection, sorted by FACS for BCR-ABL+ and NUP98-HOXA9+ (bcCML only) and retro-orbitally transplanted into cohorts of sublethally irradiated (6 Gy) C57BL/6J mice. For AML-ETOa9 and NRAS, cells were transplanted into lethally irradiated (9.5 Gy) C57BL/6J recipients along with 3 × 105 RBC lysed bone marrow rescue cells. For secondary transplants, Lin− cells from primary bcCML recipient mice were transplanted into secondary sublethally irradiated recipients. The recipients were maintained on acid water HYDROPAC and irradiated sulfatrim diet and evaluated daily. Recipients receiving additional taurine (T8691, Sigma-Aldrich) were maintained on regular chow without taurine (D10012Gi, Research Diets). Taurine was dissolved in autoclaved acid water from the HYDROPAC and supplied in sterile water bottles. For in vivo venetoclax treatments, venetoclax (ABT-199; Tocris Bioscience) solution was made fresh daily in a solvent containing 10% ethanol with 60% Phosal 50 PG and 30% PEG-400, and delivered by oral gavage at a final dose of 50 mg per kg. GES (Toronto Research Chemicals or MedChemExpress) and TAG-HCl (synthesized by Enamine) were dissolved in autoclaved acid water from the HYDROPAC and supplied in sterile water bottles (GES) or intraperitoneally (i.p.) (TAG-HCl). For in vivo rapamycin treatments, rapamycin (Selleck Chemicals) stock solution (50 mg ml−1) was made in ethanol (Sigma-Aldrich). Single-use aliquots of the stock were diluted fresh each day in vehicle containing equal parts of 10% PEG-40 with 8% ethanol and 10% Tween-80 solutions. Mice received i.p. 5 mg per kg rapamycin or vehicle from days 5–10 after transplant. Premorbid animals were euthanized at the indicated experimental timepoints or at the end point. For all experiments, mice were monitored closely for signs of disease or morbidity daily and were euthanized after visible signs of hunched dorsum, failure to thrive or any signs of infection. These limits were not exceeded for any experiment. Relevant tissues were collected and analysed by flow cytometry, RNA-seq, proteomics, metabolomics or fixed for histology. Apoptosis assays were done using annexin V and 7AAD (eBiosciences). Analysis of in vivo bromodeoxyuridine (BrdU) incorporation was performed using the APC BrdU Flow Kit (BD Biosciences) after a single i.p. injection of BrdU (2 mg at 10 mg ml−1).
RNA extraction and RT–qPCR
RNA was extracted using the RNeasy Micro or Mini kits (Qiagen) according to the manufacturer’s protocols. RNA concentrations were determined using NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). RNA quality was assessed with the Agilent Bioanalyser 2100 (Agilent Technologies). Quantitative PCR with reverse transcription (RT–qPCR) was performed on the Bio-Rad CFX96 C100 Thermocycler using Bio-Rad CFX Manager 1.1 v.4.1 (Bio-Rad) or Thermo Fisher Scientific Quant Studio 12K Flex Real Time PCR using Quant Studio v.1.2 (Thermo Fisher Scientific). RT–qPCR data were analysed using Bio-Rad CFX Manager 1.1 v.4.1 or Quant Studio v.1.2.
Isolation of mouse stromal cells for scRNA-seq and analysis
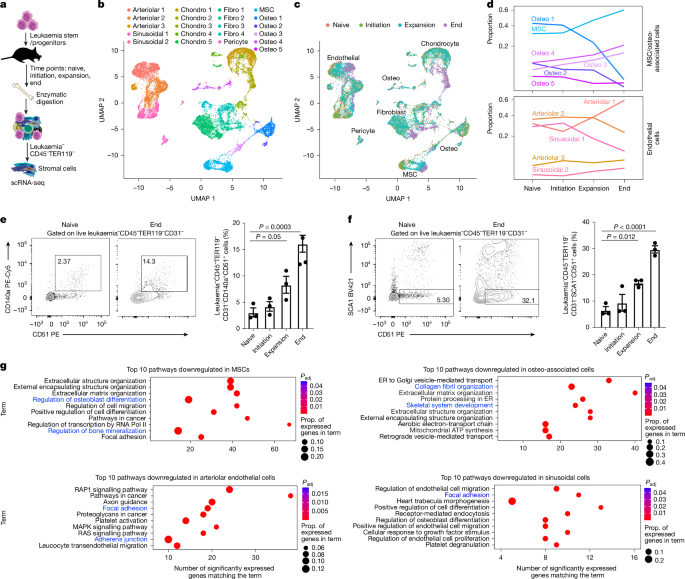
Microenvironmental populations were isolated as previously described61. In brief, bone and bone marrow were isolated from long bones and pelvis in 1× Media 199 (Gibco) with 2% FBS (GeminiBio). Bone marrow cells from 3–5 mice per timepoint were digested for 30 min in HBSS containing 2 mg ml−1 dispase II (Gibco), 1 mg ml−1 collagenase type IV (Sigma-Aldrich) and 20 ng ml−1 DNase type II (Sigma-Aldrich). Bone spicules were digested for 60 min in PBS supplemented with 2.5 mg ml−1 collagenase type I (Stem Cell Technologies) and 20% FBS. Digested bone marrow was red-blood-cell-lysed using RBC Lysis Buffer (eBioscience). Bone and bone marrow cells were pooled and CD45+TER119+ haematopoietic cells were magnetically depleted on the autoMACS cell separator (Miltenyi Biotec). The CD45−TER119− stromal cells were either stained and analysed for candidate populations by flow cytometry (BD LSRFortessa) or further enriched by sorting (BD FACSAria II) and processed for scRNA-seq.
Mouse bone marrow scRNA-seq and analysis
Cell suspensions were processed to generate scRNA-seq libraries using the Chromium Next GEM Single Cell 3′ GEM, Library and Gel Bead Kit v3.1 (10x Genomics) according to the manufacturer’s recommendations. The samples were loaded onto the Chromium Single-Cell Instrument (10x Genomics) to generate single-cell gel bead in emulsions (GEMs). GEM reverse transcription (GEM-RT) was performed to produce a barcoded, full-length cDNA from polyadenylated mRNA. After incubation, GEMs were broken, the pooled GEM-RT reaction mixtures were recovered and cDNA was purified with silane magnetic beads (DynaBeads MyOne Silane Beads, Thermo Fisher Scientific). The purified cDNA was further amplified by PCR to generate sufficient material for library construction. Enzymatic fragmentation and size selection was used to optimize the cDNA amplicon size and indexed sequencing libraries were constructed by end repair, A-tailing, adaptor ligation and PCR. The final libraries contained the P5 and P7 priming sites used in Illumina bridge amplification. Mouse samples were sequenced on the Illumina NovaSeq 6000 S2 flowcell while human samples were sequenced across several lanes of the Illumina NovaSeq X-plus 25B flowcell. The samples were demultiplexed and counted using cellranger v.4.0.0 mkfastq and count, using the default parameters. The samples were aligned to a custom reference containing the 10x provided mm10-2020-A mouse reference and an additional eGFP sequence.
Seurat v.4.1.0 within R v.4.1.1 was used for most of processing. Moreover, dplyr v.1.2.0 and tidyverse v.1.3.2 were used extensively for data piping and transformation. Samples were imported and cells were filtered for at least 200 features captured per cell and features were filtered for expression in at least 3 cells. Additional filters were applied, filtering out all cells with higher than 5% mitochondrial content and filtering out all cells positive for CD45, CD71, TER119 and eGFP. All samples were merged using ‘merge’ and normalized using SCTransform, regressing out the impact of mitochondrial features. Principal component analysis was performed using the first 40 principal components (RunPCA) and clusters were generated using FindNeighbors and FindClusters (resolution=0.5). UMAP dimensional reduction was also performed using RunUMAP using the first 30 principal components. Clusters were initially typed using scMCA v.0.2.0. Populations typed as non-stromal (B cell, pre-B cell, pro-erythrocyte, pro-erythroblast, neutrophil and megakaryocyte) were filtered from the dataset. Populations were further typed using published bone marrow stroma markers13. FindAllMarkers was used within the context of specific populations to determine which genes change significantly over time. DEGreport-implemented (v.1.30.3) degPatterns was used to tie lineage-specific expression patterns to the timepoints. Expression patterns corresponding to broadly increased or decreased expression over time were passed to gene set enrichment against KEGG_2019, GO_Biological_Process_2021, WikiPathways_2019_Mouse and ChEA_2022 databases using EnrichR-3.0.
Human bone marrow microenvironment scRNA-seq analysis
Bone marrow aspirates were obtained from patients with MDS/AML after written informed consent in accordance with the Declaration of Helsinki and approval of University of Rochester institutional review board (IRB). To isolate the bone spicules, bone marrow aspirates were passed through a 40 μm cell strainer. The filter containing spicules was washed with PBS and placed in a six-well plate. The spicules were digested for 1 h in collagenase (Stem Cell Technologies) at 37 °C. After filtering, the filtered aspirate was used to isolate bone marrow mononuclear cells by density centrifugation (Ficoll-Paque, GE Healthcare). Digested bone cells and bone marrow mononuclear cells were pooled and stained with CD45–APC (BD Biosciences) followed by staining with anti-APC microbeads (Miltenyi Biotec). The stained cells were magnetically depleted for CD45− cell fraction using LD columns (Miltenyi Biotec). The CD45− cells were processed for scRNA-seq as described above.
Samples containing cells expressing COL1A1 were integrated into a single dataset using Seurat v.4.1.0 within R v.4.1.1. Cells with mitochondrial features making up greater than 25% of the detected transcripts were removed. The samples were scaled and normalized together, regressing out mitochondrial and globin-related content. Globin-related content was determined using HBB, HBA2, HBA1, HBD and HBM. Further regression was performed using Harmony v.0.1.0 to reduce the impact of sample specific effects. From there, the standard Seurat procedure was used, clustering to a resolution of 0.8.
A normal bone marrow reference was created using GEO GSE253355 (ref. 23). Data from this submission were normalized, PCA was run using the first 50 principal components, and UMAP was run using the first 50 principal components within Seurat v.5.0.3.9911. This dataset was then used within Azimuth v.0.5.0 to create the reference using Azimuth standard methods. The samples were then typed using this reference with the RunAzimuth function against the L1, L2 and coarse annotations.
Primary human CD34+ cell RNA-seq analysis
Total RNA was purified with Qiagen RNeasy PLUS kit according to the manufacturer recommendations and eluted in nuclease-free water. The total RNA concentration was determined using the NanoDrop 1000 spectrophotometer (NanoDrop), and the RNA quality was assessed using the Agilent Bioanalyser (Agilent Technologies). The TruSeq Stranded mRNA Sample Preparation Kit (Illumina) was used for next generation sequencing library construction according to the manufacturer’s protocols. In brief, mRNA was purified from 200 ng total RNA with oligo-dT magnetic beads and fragmented. First-strand cDNA synthesis was performed with random hexamer priming followed by second-strand cDNA synthesis using dUTP incorporation for strand marking. End repair and 3′ adenylation was then performed on the double-stranded cDNA. Illumina adaptors were ligated to both ends of the cDNA, purified by gel electrophoresis and amplified with PCR primers specific to the adaptor sequences to generate cDNA amplicons of approximately 200–500 bp in size. The amplified libraries were hybridized to the Illumina single end flow cell and amplified using the cBot (Illumina). Single-end reads of 100 nucleotides were generated for each sample using Illumina’s HiSeq2500v4.
Raw reads generated from the Illumina basecalls were demultiplexed using bcl2fastq v.2.19.0. Quality filtering and adapter removal was performed using FastP v.0.20.1. Processed reads were then mapped to the human reference genome (hg38 + gencode v36; https://www.gencodegenes.org/human/release_36.html) using STAR_2.7.6a. Reads mapping to genes were counted using subread featurecounts v.2.0.1. Differential expression analysis was performed using DESeq2 v.1.28.1 with a Padj threshold of 0.05 within R v.4.0.2. Gene Ontology analyses were performed using the EnrichR-3.0 package.
Determining cell surface protein–ligand interactions
To determine significantly expressed receptors, the differential expression results from human RNA-seq described above were first filtered for significantly changing (Padj < 0.05) genes with a log-transformed fold change value of greater than 0. A list of potential cell surface receptors was generated using this gene list in conjunction with cell surface proteins detailed within the Cell Surface Protein Atlas19 and essential for LSC growth in vivo6. The resultant gene list was further reviewed for genes that are not truly expressed within the cell surface, leading to the removal of CHST11, ST3GAL4, ACAA1, CLCN6, CHPF2, CTSK, ATP6AP1, CTSD, PNPLA6, DMXL2, TUBB6, MAN2B2, CLN3, MGAT4B, MYH9 and PIGG from further review. Ligand expression from the temporal scRNA-seq dataset corresponding to bcCML and AML expressed receptors was determined by differential expression across the populations. Differentially expressed genes were filtered for corresponding ligands using a combination of nichenetr (v.1.1.0)62 and literature-supported interactions. These included NRP163, ADSL64,65, CDO1 and CSAD for taurine synthesis66, GADL1 and CNDP1 for β-alanine synthesis67,68, KIT69, CD15570 and CD3371 for those not captured within this mapping. The Broad Institute hosted dataset20 was used as a stand in for healthy immune microenvironment within the context of AML. Counts (RNA_soupX1.mtx.gz) and metadata (metadata_clustering_w_header_upd.csv) were downloaded from the Broad Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/study/SCP1987/an-inflammatory-state-remodels-the-immune-microenvironment-and-improves-risk-stratification-in-acute-myeloid-leukaemia) and imported using Seurat. FindMarkers was used to determine which genes were upregulated within the microenvironment in relation to the other populations. Significantly expressed genes were then filtered for ligands using the same method as the stromal microenvironment. Significantly expressed ligand and receptor mappings within the stroma microenvironment, the immune microenvironment and within bcCML and AML human bulk RNA-seq data were visualized using circlize v.0.4.15. For illustration purposes, ligands expressed with a log-transformed fold change of 5 or higher were limited to 5.
Mouse Slc6a6 wild-type and knockout leukaemia RNA-seq
The RNeasy Plus Micro Kit (Qiagen) was used for RNA extraction. RNA concentration was determined using the NanoDrop 1000 spectrophotometer (NanoDrop), and the RNA quality was assessed using the Agilent Bioanalyser 2100 (Agilent Technologies). A total of 1 ng of total RNA was pre-amplified using the SMARTer Ultra Low Input kit v4 (Clontech) according to the manufacturer’s recommendations. The quantity and quality of the subsequent cDNA was determined using the Qubit Fluorometer (Life Technologies) and the Agilent Bioanalyser 2100 (Agilent). Then, 150 pg of cDNA was used to generate Illumina-compatible sequencing libraries using the NexteraXT library preparation kit (Illumina) according to the manufacturer’s protocols. The amplified libraries were hybridized to the Illumina flow cell and sequenced using the Illumina NextSeq 550 (Illumina). Single-end reads of 100 nucleotides were generated for each sample. The mouse bulk RNA-seq samples were processed otherwise identical to the human bulk RNA-seq with two exceptions: m38 + gencode M27 reference (https://www.gencodegenes.org/mouse/release_M27.html) for use within alignment and counting, and ‘-s 0’ being used within subread featureCounts. See the ‘Primary human CD34+ cell RNA-seq analysis’ section for details on differential expression analysis.
Primary human patient-derived cells and human leukaemia cell lines
For human RNA-seq experiments and other studies, CD34+ cells were isolated from bone marrow samples of healthy donors and samples from patients with AML and bcCML obtained under University of Rochester institutional review board-approved protocols with written informed consent in accordance with the Declaration of Helsinki. The normal human CD34+ HSPCs used in all functional assays were purchased (Stem Cell Technologies). Cells were cultured in Iscove’s modified Dulbecco’s medium with 10% FBS, 100 IU ml−1 penicillin–streptomycin (Gibco) and 55 μM 2-mercaptoethanol, 1× LDL (Sigma-Aldrich) and supplemented l-glutamine with 100 ng ml−1 SCF and TPO (R&D Systems). Human leukaemia cell lines K562, THP1 and M-V-411 (ATCC) were maintained in RPMI/IMDM with 10% FBS, 100 IU ml−1 penicillin–streptomycin (Gibco). These cell lines were validated by vendor. MDS-L cells (from K. Tohyama) were authenticated in house by flow cytometry as CD45+CD34+CD38+ and maintained in RPMI with 10% FBS supplemented with 20 ng ml−1 IL-3 (PeproTech). Cell lines were not tested for mycoplasma. For colony-forming assays with shRNAs, leukaemia and normal cells were transduced with the indicated lentiviral shRNAs. Cells were sorted 24 h after infection and plated in CFU assays or transplanted in sublethally irradiated NSG mice.
MSC isolation, osteogenic differentiation and co-culture with leukaemia cells
MSCs were isolated from leukaemic mice and cultured in 10 cm dishes in MEMα with no ascorbic acid (Gibco) supplemented with 15% FBS and 100 IU ml−1 penicillin–streptomycin (Gibco). Then, 6 days after culture initiation, the cells were sorted for CD45−CD3−B220−TER119−GR1−CD31−CD51+SCA1+ MSCs. Sorted cells were expanded in the medium descried above. For co-culture experiments, 50,000 MSCs were plated in a 48-well plate, and transduced with the indicated lentiviral shRNAs. Then, 3 days after infection, osteogenic differentiation was induced by switching to MEMα (Gibco) supplemented 15% FBS, 100 IU ml−1 penicillin–streptomycin (Gibco) along with 50 μg ml−1 ascorbic acid (Sigma-Aldrich), 10 mM β-glycerolphosphate (Sigma-Aldrich) and 100 nM dexamethasone (Sigma-Aldrich) for 6 days. On day 6 after differentiation, 100,000 Lin− bcCML cells were added in X-Vivo supplemented with 10% FBS, 50 μM 2-mercatpoethanol and penicillin–streptomycin. Then, 3 days after co-culture, leukaemia cells were analysed for cell viability and plated in methylcellulose for colony-forming assays. Microscopy images were obtained on THE Olympus CKX41 SYSTEM using CellSens Entry v.2.3 (Olympus).
Normal HSC in vivo transplantation assays
For bone marrow transplants, 500 HSCs were isolated from bone marrow of Slc6a6+/+ or Slc6a6−/− mice and transplanted into lethally irradiated (9.5 Gy) CD45.1 mice along with 2 × 105 SCA1-depleted bone marrow rescue cells. For subsequent secondary transplants, 2 × 106 red-blood-cell-lysed bone marrow cells isolated from primary recipient mice were transplanted into lethally irradiated (9.5 Gy) CD45.1 mice. Peripheral blood of recipient mice was collected every 4 weeks for 4 months after transplant and bone marrow analysed at the end of 4 months.
Methylcellulose colony-formation assays
For colony assays with mouse cells, the indicated numbers of Lin− bcCML cells or KIT+ AML cells were plated in methylcellulose medium (M3234, StemCell Technologies). Colonies were scored at 7 days. For colony assays with human cell lines or patient-derived AML samples, cells were plated in methylcellulose medium (H4434; StemCell Technologies). Colony numbers were counted 10–14 days after plating. Taurine antagonist or TAG (synthesized by Enamine; 92% pure), GES (G827500, Toronto Research Chemicals), taurine (T8691, Sigma-Aldrich), mTOR activator (MHY1485, Sigma-Aldrich) and Venetoclax (ABT-199, Tocris Bioscience) were used as described. Venetoclax and GES Synergy quantification was calculated using Chou-Talalay method72.
Seahorse assays
The Seahorse XF Glycolysis Stress Test Kit (Agilent Technologies, 103020-100) and the Seahorse XF cell mito stress test kit (Agilent Technologies, 103015-100) were used to measure glycolytic flux (ECAR) and oxygen consumption (OCR) respectively. Mouse Lin− bcCML cells were sorted and cultured for 48 h in X-Vivo supplemented with 10% FBS, SCF and TPO. Then, 1 h before the analysis, 50,000–100,000 cells were seeded in Cell-Tak-coated (Corning, 324240) 96-well XF96 well plates in Seahorse XF medium (Agilent Technologies, 102353-100), and the plate was incubated at 37 °C. ECAR data were measured after sequential addition of glucose (10 mM), oligomycin (1μM) and 2-deoxyglucose (50 mM) using the XF96 analyser (Agilent Technologies). OCR data were measured after sequential addition of oligomycin (1.5 μM), carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP) (0.5 μM), rotenone and antimycin (0.5 μM) using the XF96 analyser. Data were analysed using Wave v.2.6.3 (Agilent Technologies).
Western blot analysis
Cell lysates prepared in 1× RIPA (Thermo Fisher Scientific) supplemented with 1× protease, 1× phosphatase inhibitors (Cell Signaling Technology) and 250 IU benzonase nuclease (Millipore Sigma) were separated on gradient polyacrylamide gels and transferred to nitrocellulose blotting membrane (0.45 μM; GE Healthcare). Primary antibodies against phosphorylated mTOR, mTOR, phosphorylated S6K, S6K, pEIF4B, EIF4B and β-actin (Cell Signaling Technology) or tubulin (Abcam) were used. Horse-radish peroxidase (HRP)-conjugated anti-rabbit antibodies (Cell Signaling Technology) were used to detect primary antibodies. Immunoblots were developed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific). Immunoblots were imaged using the LI-COR Odyssey M system using Empiria Studio v.2.3 (LI-COR). Images were analysed using Empiria Studio v.3.2.0.186 (LI-COR). Raw gel images are provided in Supplementary Fig. 1.
Protein extraction, sample preparation and MS analysis
Protein extraction
Cells were lysed in 50 μl of 5% SDS, 100 mM triethylammonium bicarbonate (TEAB, Thermo Fisher Scientific) and 50 μg of protein from each sample was reduced with dithiothreitol (Sigma-Aldrich). Proteins were alkylated and trapped to S-Trap micros (Protifi), digested with trypsin and extracted by sequential additions of 0.1% trifluoroacetic acid (TFA) in acetonitrile. Then, 1% of each sample was used for global DIA analysis, and the remaining sample was frozen and dried down in the Speed Vac (Labconco) before TMT labelling. The samples were reconstituted in TEAB and labelled with TMT 10-plex reagents (Thermo Fisher Scientific) according to the manufacturer’s protocol. All of the samples were combined and dried down in a speed vac before desalting with 0.1% TFA in acetonitrile using the 130 mg C18 sorbent sep-pak attached to a 3 ml syringe (Waters). Desalted samples were frozen and dried down before phosphorylation enrichment using the High-Select FeNTA Enrichment Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Enriched phosphorylated samples were frozen, dried down and fractionated using C18 spin columns. The fractions were eluted by stepwise addition of 10 mM AmmOH with increasing acetonitrile concentrations: 3.5, 6.5, 9.5, 12.5, 15.5, 18.5, 27, 50%. The eight fractions were concatenated to four by combining fractions 1/5, 2/6, 3/7, 4/8. Fractionated samples were frozen, dried down and reconstituted in 0.1% TFA for MS analysis.
MS analysis
Non-TMT-tagged peptides were injected onto a 75 μm × 2 cm trap column before refocusing on a 100 μm × 15 cm C18 column with 1.8 μm beads (Sepax) using the Vanquish Neo UHPLC (Thermo Fisher Scientific) system connected to the Orbitrap Astral mass spectrometer (Thermo Fisher Scientific). Ions were introduced to the mass spectrometer using a Nanospray Flex source operating at 2 kV. Solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in 80% acetonitrile) formed the gradient starting at 1% B and ramped to 99% B for a total runtime of 14 m. After each run was completed, the column was re-equilibrated with 1% B before the next injection. The Orbitrap Astral was operated in data-independent acquisition (DIA) mode for global proteomic analysis, with MS1 scans acquired at a resolution of 240,000 with a maximum injection time of 5 ms over a range of 390–980 m/z. DIA MS2 scans were acquired using 2 Da windows, a 3 ms maximum injection time, an HCD collision energy of 25% and a normalized automatic gain control (AGC) of 500%. Fragment ions were collected over a scan range of 150–2,000 m/z. Phosphoprotein analysis was carried out on the same Vanquish Neo UHPLC and Orbitrap Astral mass spectrometer system, but with several changes to the LC and instrument settings. To account for ratio compression inherent with TMT, samples were high-pH fractionated to reduce complexity before MS analysis. A longer gradient of 5–30% B over 48 min was used to further separate phosphopeptides. A data-dependent acquisition method using a FAIMS Pro Duo (Thermo Fisher Scientific) was used with three compensation voltages (−40 V, −60 V, −80 V) to further reduce the sample complexity. For each CV, a full scan was acquired over a range of 400–1,500 m/z in the Orbitrap, while MS2 scans were analysed in the Astral analyser for 1 s, after which the instrument switched to the next CV and the process was repeated for a total cycle time of 3 s. Peptides with a charge state of between 2 and 6 were isolated based on intensity with a 0.5 Da isolation window, and were fragmented with an HCD collision energy of 35%. The maximum injection time was 20 ms, and the normalized AGC target was 100%. Dynamic exclusion was set to 15 s.
Data analysis
The global DIA raw data were processed with DIA-NN v.1.8.1 (https://github.com/vdemichev/DiaNN) using library-free analysis mode. The library was annotated using the Mus musculus UniProt database (UP000005640_9606). For precursor ion generation, the maximum number of missed cleavages was set to 1, maximum number of variable modifications to 1 for Ox(M), peptide length range to 7–30, precursor charge range to 2–3, precursor m/z range to 380–980, and fragment m/z range to 150–2,000. The quantification was set to ‘Robust LC (high precision)’ mode with RT-dependent median-based cross-run normalization enabled, MBR enabled, protein inferences set to ‘Genes’ and ‘Heuristic protein inference’ turned off. Precursors were filtered at library precursor q-value (1%), library protein group q-value (1%) and posterior error probability (50%). Protein quantification was carried out using the MaxLFQ algorithm (https://github.com/vdemichev/diann-rpackage) and the number of peptides quantified in each protein group was determined using the DiannReportGenerator Package (https://github.com/URMC-MSRL/DiannReportGenerator). Further filtering, missing value imputation and statistical tests were performed using Perseus73. Phosphoproteome raw data were searched using the CHIMERYS within the Proteome Discoverer software platform v.3.1 (Thermo Fisher Scientific), allowing for up to two missed cleavages, with a fragment mass tolerance of 10 ppm. Carbamidomethyl on cysteine, and TMT on lysine and peptide N terminus were set as fixed modifications, while oxidation of methionine and phosphorylation of serine, threonine and tyrosine were set as variable modifications. Reporter ions were quantified using the Reporter Ions Quantifier node, with an integration tolerance of 20 ppm, and the integration method was set to ‘most confident centroid’.
Immunohistochemical staining and analysis
Paraffin-embedded 4 μm human bone marrow biopsy sections were deparaffinized in xylene and antigen epitopes were retrieved using BOND epitope retrieval solution (pH 9) for 10 min. Endogenous peroxidases were quenched with a 10 min incubation in 3% H2O2-methanol solution. The sections were then blocked in 10% donkey serum for 60 min. The sections were either incubated with anti-CDO1 (Proteintech) overnight or with anti-osterix (Abcam) for 2 h at room-temperature. The sections were washed in PBS and stained with HRP-conjugated donkey anti-rabbit secondary antibody (Jackson Immunoresearch) for 1.5 h at room temperature. After three washes, colour was developed using the ImmPACT DAB Substrate kit (Vector laboratories, SK-4105) according to the manufacturer’s protocol. The sections were then counterstained with haematoxylin. Three different areas of each section were imaged on the Olympus BX41 microscope with a ×20 (0.5 NA) objective. Images were analysed using the IHC plugin toolbox in Fiji v.1.54g.
Immunofluorescence staining
Leukaemia cells were cultured in RPMI without amino acids (US Biologicals) in the presence or absence of taurine (Sigma-Aldrich) and seeded on eight-well cover glass chambers (BD biosciences) coated with Cell-Taq (Corning) according to the manufacturer’s protocols. MSCs were grown and differentiated in 35 mm glass-bottom dishes with 14 mm microwell (MatTek Life Sciences). Cells were fixed with 4% PFA and blocked in blocking buffer (PBS with 5% donkey serum, 1% BSA and 0.1% Triton X-100) and incubated overnight in primary antibodies diluted in the blocking buffer. Primary antibodies used included mTOR (Cell Signaling Technologies), LAMP1 (DHSB) or CDO1 (Proteintech). Cells were washed in PBS containing 0.1% Tween-20 (Sigma-Aldrich), stained with Alexa-Fluor-conjugated secondary antibodies (Thermo Fisher Scientific), and mounted in Fluormount G (Thermo Fisher Scientific).
Immunofluorescence imaging and analysis
Immunofluorescence images were acquired with the Teledyne Photometrics Prime BSI express sCMOS camera mounted on the Nikon ECLIPSE Ti2 inverted microscope equipped with the NIS-Elements 6D imaging acquisition module (v.5.42.06). The Nikon D-LEDI fluorescence LED illumination system (equipped with 385 nm, 488 nm, 568 nm and 621 nm excitation wavelengths) was used as the primary illumination source. Specific illumination wavelengths were selected by combining a large field of view quad-filter cube (DAPI/FITC/TRITC/CY5; 96378) with specific Lumencor emission filters (FF01-474/27-32, FF01-515/30-32, FF01-595/31-32, FF02-641/75-32). MSC and osteolineage cultures were imaged with the Nikon CFI60 Plan Apochromat Lambda D ×20 (0.8 NA) objective lens, and leukaemia cells were imaged using the Nikon CFI60 Plan Apochromat Lambda D ×100 (1.45 NA) objective lens. Images were deconvoluted using Imaris v.10.2 (Oxford instruments), and Pearson’s co-localization analysis was performed using the JACoP BIOP plugin in Fiji v.1.54g.
Phosphoflow cytometry
Lineage depleted Slc6a6+/+ and Slc6a6−/− mouse leukaemia cells were fixed using BD Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Bioscience) according to the manufacturer’s protocols. Cells were stained with primary antibodies against phosphorylated mTOR (Cell Signaling Technology). Cells were then stained with donkey anti-rabbit secondary antibody conjugated with Alexa Fluro 488 (Invitrogen) to detect p-mTOR. Analysis was performed on the LSRFortessa (BD Biosciences) system. Data were analysed using FlowJo software.
Taurine quantification
Bone marrow cells or bone marrow peripheral fluid were isolated from femurs in Hanks’ balanced salt solution (Gibco) with 5% FBS and 2 mM EDTA. For taurine analysis of bone marrow cells after genetic loss of Slc6a6 or treatment of leukaemia cells with taurine inhibitor treatments, cells were lysed in RIPA buffer (Thermo Fisher Scientific) with benzonase nuclease (Sigma-Aldrich). For bone marrow interstitial fluid analysis, one femur was crushed in 1 ml of buffer, filtered and centrifuged at 1,500g for 5 min. The supernatant was concentrated using 10,000 MWCO spin columns (Corning). Then, 25 μl of concentrated samples was quantified using the Taurine Assay kit (Sigma-Aldrich/Abcam) according to the manufacturer’s protocols on the BioTek Synergy 2 plate reader using Gen5 v.3.11 (BioTek). The samples were corrected for taurine amounts in unconditioned fresh medium or buffer. Alternatively, cell pellets were processed as described in the liquid chromatography–mass spectrometry (LC–MS) section below, and taurine levels were measured by LC–MS (Orbitrap Exploris 240).
Untargeted metabolomics of Lin− leukaemia cells
For untargeted metabolomics, spleens from mice bearing Slc6a6+/+ or Slc6a6−/− leukaemias were quickly dissected and dissociated in Hanks’ balanced salt solution (Gibco) with 5% FBS and 2 mM EDTA at 4 °C. Leukaemia cells were collected and maintained at 4 °C all through the process of isolation, staining and magnetic sorting to minimize metabolic changes. Lin+ (CD3ε−CD4−CD8−GR1−CD11b−TER119−CD45R−CD19−) leukaemia cells were magnetically depleted using LD columns (Milteny Biotec). Lin− leukaemia stem cell fractions were washed with PBS containing 5 mM glucose and centrifuged at 3,000g for 1 min, snap-frozen and processed for metabolomics as described in the ‘LC–MS analysis’ section below.
13C-taurine tracing in leukaemia cells
K562 cells were cultured in serum-free RPMI-1640 medium in six-well plates for 48 h. The cells were then cultured in serum free RPMI-1640 supplemented with 200 μM taurine (1,2 13C2, 98%; Cambridge Isotope Labs) or no additional taurine for 24 h. The cells were washed with PBS containing 5 mM glucose and centrifuged at 3,000g, snap-frozen and processed for metabolomics as described below in the ‘LC–MS analysis’ section.
LC–MS analysis
Frozen cell pellets were resuspended at 2 million cells per 1 ml of 80% methanol by vortexing, transferred to −80 °C for 30 min and then to regular ice for 30 min with vortexing every 10 min. Next, the samples were centrifuged at 17,000g for 10 min and 90% of supernatant was dried down in a vacuum evaporator (Thermo Fisher Scientific). The samples were reconstituted in 50% acetonitrile (A955, Thermo Fisher Scientific) at a volume equal to 10% of the dried down volume and transferred to glass vials for LC–MS analysis. The metabolite extracts were analysed by high-resolution MS with the Orbitrap Exploris 240 (Thermo Fisher Scientific) system coupled to the Vanquish Flex LC system (Thermo Fisher Scientific). Then, 2 µl of the samples was injected onto the Waters XBridge XP BEH Amide column (150 mm length × 2.1 mm inner diameter, 2.5 µm particle size) maintained at 25 °C, with a Waters XBridge XP VanGuard BEH Amide (5 mm × 2.1 mm inner diameter, 2.5 µm particle size) guard column. For positive-mode acquisition, mobile phase A was 100% LC–MS-grade H2O with 10 mM ammonium formate and 0.125% formic acid. Mobile phase B was 90% acetonitrile with 10 mM ammonium formate and 0.125% formic acid. For negative-mode acquisition, mobile phase A was 100% LC–MS-grade H2O with 10 mM ammonium acetate, 0.1% ammonium hydroxide and 0.1% medronic acid (Agilent). Mobile phase B was 90% acetonitrile with 10 mM ammonium acetate, 0.1% ammonium hydroxide and 0.1% medronic acid. The gradient was 0 min, 100% B; 2 min, 100% B; 3 min, 90% B; 5 min, 90% B; 6 min, 85% B; 7 min, 85% B; 8 min, 75% B; 9 min, 75% B; 10 min, 55% B; 12 min, 55% B; 13 min, 35% B; 20 min, 35% B; 20.1 min, 35% B; 20.6 min, 100% B; 22.2 min, 100% B; all at a flow rate of 150 μl min−1, followed by 22.7 min, 100% B; 27.9 min, 100% B at a flow rate of 300 μl min−1, and finally 28 min, 100% B at flow rate of 150 μl min−1, for a total length of 28 min. The H-ESI source was operated in positive mode at spray voltage 3,500 V or negative mode at spray voltage 2,500 V with the following parameters: sheath gas 35 au, aux gas 7 au, sweep gas 0 au, ion transfer tube temperature 320 °C, vaporizer temperature 275 °C, mass range 70 to 1,000 m/z, full scan MS1 mass resolution of 120,000 FWHM, RF lens at 70% and standard AGC. LC–MS data were analysed using Compound Discover (v.3.3, Thermo Fisher Scientific) and El-Maven software74 for peak-area determination and compound identification. Compounds were identified by matching to LC–MS method-specific retention time values of external standards and MS2 spectral matching to external standards and the mzCloud database (Thermo Fisher Scientific). Raw P values were calculated using pairwise Mann–Whitney–Wilcoxon rank-sum tests and Padj values were computed using Benjamini–Hochberg false-discovery rate correction. Data were uploaded to the Metabolomics Workbench75.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software v.6.0 (GraphPad). Data are mean ± s.e.m. One-way ANOVA, two-way ANOVA, unpaired two-sided Student’s t-tests, multiple unpaired t-tests corrected with the Benjamin–Hochberg method, ratio-paired t-tests, and log-rank tests were used to determine statistical significance. Combination index and isobologram plots were made using CompuSyn72.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.