Cell lines and reagents
The mouse Ba/F3 parental cell line was obtained from the Dana-Farber Cancer Institute in 2003. Cells were maintained in RPMI 1940 GlutaMAX medium (ThermoFisher, 61870-036) supplemented with 10% (v/v) fetal bovine serum (FBS) (Corning, 35-015-CV), 2 mM sodium pyruvate (BioConcept Amimed, 5-60F00-H), 10 mM HEPES (BioConcept, 5-31F00-H) and 5 ng ml−1 recombinant murine IL-3 (Life Technologies, PMC0035). Derived Ba/F3 KRAS and NRAS mutants have been cultured in IL-3 withdrawal. Mel Juso, IPC298 and SKMEL30 cell lines were obtained from the DMSZ. VMM39, HT-1080, Hep G2, NCI-H358, Miapaca2, SW1990, A375, NCI-H1437, Panc04.03 and Calu-6 were obtained from ATCC. The MM485 cell line was obtained from The CellBank Australia. UACC-257 was obtained from the US National Cancer Institute. The KP4 cell line was obtained from the RIKEN cell bank. The PC9 cell line was obtained from K. Nishio at the Department of Genome Biology, Kinki University School of Medicine. The 293FT was obtained from Invitrogen. The Mel Juso IPC298, SK-MEL-30, MM485, VMM39, Calu-6, UACC-257, NCI-H358, SW1990, PC9, NCI-H1437 and Panc04.03 cell lines were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS) (Corning, 35-015-CV). The HT-1080, Miapaca2, A375, KP4 and 293FT cell lines were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% (v/v) FBS (Corning, 35-015-CV). The HepG2 was maintained in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% (v/v) FBS (Corning, 35-015-CV). All cell lines were cultured according to the standard instructions provided by the suppliers (DSMZ, CellBank Australia, ATCC, Invitrogen, RIKEN and NCI).
Generation of stable lines
Stable lines expressing SHOC2, NRAS non-targeting shRNAs were generated by lentiviral infection. The lentivirus production was performed first using HEK293FT cells. Cells were seeded in T175 cell-culture flasks and transfected with 12 µg pVPR-Gag-Pol, 4.8 µg pMD2-VSV-G and 1 µg of a lentiviral vector expressing shRNA using Mirus TransIT (Mirus, MIR2700). The virus-containing supernatant was collected and filtered using 0.45-µm syringe filters 48 h later and was subsequently used for transduction of cancerous cell lines. Then, 24 h after infection, cells were selected with puromycin (Sigma, P9620).
Lentiviral shRNA constructs
The shRNA Dox-inducible constructs were generated by cloning shRNA into a pLKO-tetON-puro backbone (Addgene, 21915) using AgeI or EcoRI restriction sites. The target sequences for the different shRNAs are shRNA SHOC2-169012 (GCTTGAGTCACCATGAGTAGT), shRNA SHOC2-169009 (CTGACTCTCTTGATAACTTGA), shRNA NRAS (CCATGAGAGACCAATACATGA) and shRNA non-targeting (GGATAATGGTGATTGAGATGG).
Immunoblotting
Cells were rinsed with ice-cold PBS, pelleted and snap frozen at −80 °C. Whole-cell lysates were prepared with lysis buffer (1% Triton X-100, 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA) supplemented with a PhosSTOP phosphatase inhibitor cocktail tablet (Sigma, 4906845001) and cOmplete, EDTA-free protease inhibitor cocktail tablet (Sigma, 4693159001).
Cell lysates were cleared by centrifugation at 13,000 rpm at 4 °C for 20 min, and protein concentration was determined using the BCA protein assay kit (Thermo Scientific, 23225). Samples were normalized for protein content, denatured by the addition of Laemmli buffer (Bio-Rad, 1610747) and boiling for 5 min, and then loaded on Criterion TGX 4–15% Precast gel (Bio-Rad, 5671085). Proteins were transferred to Trans-Blot Turbo Midi Nitrocellulose Transfer Packs membrane (Bio-Rad, 1704159) using the Trans-Blot Turbo system (Bio-Rad, 1704150). All membranes were blocked with 5% milk (Sigma, 70166) and then incubated overnight with primary antibodies. Immunoblots were imaged using the FUSION FX7 imaging system (VILBER), and densitometry analysis was done using its integrated quantification software.
Antibody list
All antibodies were commercially sourced and used according to manufacturer instructions. NRAS antibody was purchased from Calbiochem (ref. OP25). RAS antibody was purchased from Abcam (Ab108602). SHOC2 (53600), Phospho-c-Raf Ser259 (9421), GFP (2956), phospho-p44/42 MAPK (Erk1/2), Thr202/Tyr204 (4370), p44/42 MAPK (Erk1/2) (9102), phospho-MEK1/2 Ser217/221 (9154), phospho-Akt Thr308 (9275), phospho-Akt Ser473 (9271), phospho-S6 ribosomal protein Ser235/236 (2211), phospho-S6 ribosomal protein Ser240/244 (Ref. 2215), phospho-IGF-I receptor β Tyr1131 (3021), IGF-I receptor β (9750), Cas9 HRP conjugate (97982), HRP-linked mouse IgG (7076) and HRP-linked rabbit IgG (7074) antibodies were purchased from Cell Signaling Technology. The FLAG HRP conjugate (A8592), vinculin (V9131) and α-tubulin (T6199) antibodies were purchased from Sigma-Aldrich.
Active RAS pull-down assay
Levels of active GTP-loaded RAS were determined using a RAS activation assay biochem kit (Cytoskeleton, BK008). Mel Juso and A347 cells were treated with 30 µM of compound 6 or an equivalent volume of DMSO for 2 h and 4 h. Cells were lysed following the manufacturer’s instructions. Pull-down was performed with 400 µg of lysate mixed with 30 µl of Raf-RBD beads and incubated on a rotator for 1 h at 4 °C. Bound proteins were eluted with 2× Laemmli buffer and boiled for 5 min at 95 °C before western blots.
Ba/F3 cell-line generation
Murine Ba/F3 cells were primarily transduced with a lentiviral Cas9 construct expression pNGx_CMV_FLAG-Cas9. Cas9-positive cells were assessed by fluorescence-activated cell sorting (FACS) and immunoblotting. Ba/F3-Cas9 cells were subsequently stably transfected with pcDNA3.1(+) EGFP-T2A-FLAG -KRAS G12C, G12D, Q61R, Q61H or NRAS G12C, G12D and Q61R. All Ba/F3 transfections were done by electroporation. For each transfection, 2 million cells were collected, pelleted and washed in PBS without Mg2+ or Ca2+. Washed cells were then resuspended in 100 µl of buffer R per sample and distributed to each DNA dilution containing 10 µg of related plasmid. Following the manufacturer’s instructions, electroporation was done using a NEON station set-up of 1635 V, 20 ms, 1 pulse for each sample. Cells were then left to recover overnight in six-well plates filled with pre-warmed media. Then, 48 h after electroporation, the medium was replaced with selection medium containing 600 µg ml−1 neomycin for Ba/F3-Cas9 and 600 µg ml−1 neomycin and 20 µg ml−1 blasticidin for all KRAS- or NRAS-expressing mutants. During generation, cells were kept in the presence of 5 ng ml−1 recombinant IL-3. Validation of the KRAS- or NRAS-expressing lines was done following IL-3 withdrawal, to ensure the dependency of the cells on oncogene expression.
CRISPR KO experiments
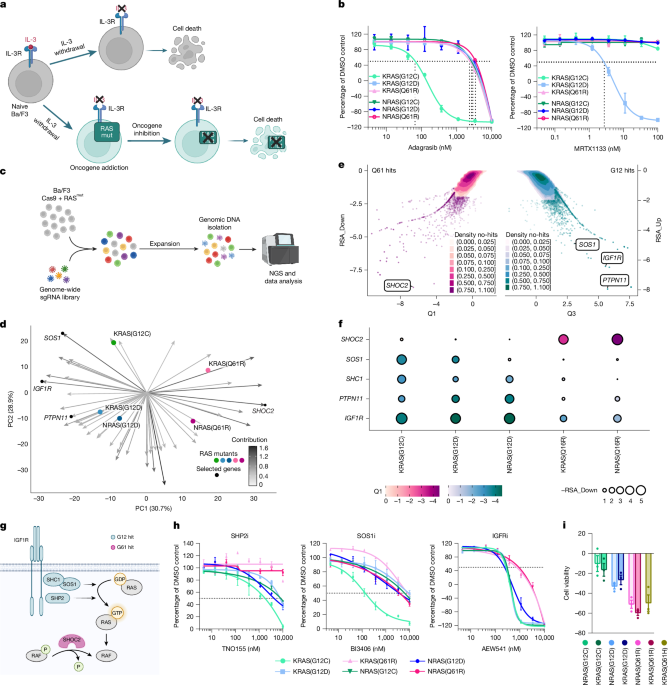
In the genome-wide isogenic screen, each of the previously engineered murine Ba/F3 cells expressing Cas9 and a single mutation in either KRAS (G12C, G12D, Q61R or Q61H) or NRAS (G12C, G12D or Q61R), as well as the control line Ba/F3 wt-Cas9, were expanded in the relevant medium and infected with either pool 1 or pool 2 of the murine genome-wide sgRNA library (Cellecta mCRISPR v.1 pool1 and pool2)38. Then, 24 h after infection, selection with 1 µg ml−1 puromycin occurred. Ba/F3 cells expressing Cas9 and Ba/F3 transduced with the KRAS or NRAS mutants were passaged in medium supplemented (or depleted) with recombinant murine IL-3. Next, 14 days after infection, the surviving cells were subjected to sample collection (duplicates) and genomic DNA preparation for deep sequencing. Raw sequencing reads were aligned using Bowtie39 with no mismatches allowed and counts were generated. Differential sgRNA representation was calculated using the DESeq2 package40. For gene-based hit calling, the sgRNAs were ranked by the robust z-score, and the statistical significance was calculated for each gene enriched towards higher rank (RSA up) and lower rank (RSA down) using the Redundant siRNA Activity (RSA) algorithm41.
Ba/F3 cell-viability assay after SHOC2 KO
The previously engineered murine Ba/F3 cell line expressing Cas9 and a single mutation in either KRAS (G12C, G12D, Q61R or Q61H) or NRAS (G12C, G12D or Q61R) were infected with either non-targeting/control sgRNA (5′ CRISPR RNA sequence: GCGAGGTATTCGGCTCCGCG) or SHOC2 sgRNA (5′ CRISPR RNA sequence: CAGGCCAAGGCGATTTAAAC). After one day of antibiotic selection, cells were seeded in 96-well plates in growth medium. The viability of the cells was assessed two days after seeding by CellTiter-Glo Luminescent Cell Viability Assay (Promega, G7572), according to the manufacturer’s instructions.
DepMap data
To evaluate screening hits in DepMap, Ceres scores from release 22Q2 were downloaded from the DepMap portal (https://depmap.org/portal/) and mean scores for each gene were calculated between cell lines bearing aberrations in G12 versus Q61 residues of HRAS, KRAS and NRAS. Statistics were calculated using t-tests corrected for multiple testing using the Benjamini–Hochberg method.
Colony formation assay
Cells were seeded in six-well plates and allowed to attach overnight in growth medium. They were then treated with 100 ng ml−1 doxycycline hydrochloride (Fisher Bioreagent, BP26535) for 10–15 d. During the assay, the cell medium was renewed every three days with doxycycline. Subsequently, the cells were fixed with 20% glutaraldehyde for 10 min, washed with water and stained with crystal violet for 30 min. The cells were then washed with water, dried and scanned. Crystal violet was then dissolved in 10% acetic acidic for 15 min. The solution was diluted 1:4 in acetic acidic and transferred to a 96-well plate in triplicate for quantification. The absorbance was read at a wavelength of 590 nm with a plate reader (BertholdTech Mithras).
Incucyte cell proliferation
Cells were seeded (in sextuplicate) in ultralow attachment microplates from Corning (384-well plate, transparent round bottom, 3830) in growth medium containing 10% FCS, then centrifuged for 3 min at 200g and allowed to form spheroids or aggregates for 24 h. Cells were then treated with 30 µM of compound 6 or DMSO and transferred in an Incucyte SX5 live-cell analysis system (Satorius, Essen Bioscience), placed inside a conventional tissue-culture incubator at 37 °C with 5% CO2. The aggregates or spheroids were imaged every six hours for three weeks at a magnification of ×10. During the whole assay, the growth medium containing DMSO or compounds was renewed twice per week. Images were analysed using the Incucyte Spheroid Analysis Software Module (Sartorius, Essen Bioscience, 9600-0019) in which virtual masks were created to delineate the spheroids. The area of each spheroid for each time point was generated. The radius and the volume of the spheroids were calculated from the spheroid area (radius = √(area/π); volume = 4/3 × π × radius3). The volume data were normalized to the initial volume of each spheroid.
Quantitative PCR
Total RNA was extracted from cells using the Qiagen Rneasy kit (Qiagen, 74104) according to the manufacturer’s instructions. Quantitative PCR (qPCR) analysis was performed using Quantitec PCR MasterMix multiplex (Qiagen, 204643) on a QuantStudio 6 Real-Time PCR System (Applied Biosystems). Each qPCR reaction was performed in triplicate, using 10 ng RNA per reaction. The results were analysed using the 2 − ΔΔCt method and standardized with Actin B. All specific qPCR primers were purchased from Invitrogen (TaqMan assay): SHOC2 (Hs00201309_m1), NRAS (Hs00180035_m1), SPRY4 (Hs00229610_m1), EGFR1 (Hs00152928_m1), DUSP5 (Hs00244839_m1) and DUSP6 (Hs01044001_m1).
RNA-seq
Total RNA from cell lines or tumours was extracted using the Qiagen RNeasy Minikit. Sequencing was done using the NovaSeq 6000 platform (2 × 101 base pair reads). For the IPC-298 cell line, Illumina NovaSeq control software v.1.6.0, RTA v.3.4.4, BCL2FASTQ v.2.20.0.422 and FASTQC v.0.11.7 were used. For the Mel Juso cell line, Illumina NovaSeq control software v.1.8.1, RTA v.3.4.4, BCL2FASTQ v.2.20.0.422 and FASTQC v.0.11.9 were used. Transcript alignment and quantification were performed using PISCES v.2018.04.112 and referenced to the hg38 human genome. Differential expression analysis was performed using DESeq2 (ref. 40). In the IPC-298 cell line, for each of the four shRNAs (two shRNA targeting SHOC2, one shRNA targeting NRAS and one non-targeting shRNA), doxycycline treatment was contrasted with no doxycycline. Pre-ranked gene-set enrichment analysis was performed for each contrast using the R package fgsea with signed P-values (sign(log2(FC)) × –log10(P-value)) as ranks. Tested gene sets were obtained from the Molecular Signatures Database (MSigDB). The following pathways were tested for enrichment: MEK_UP.V1_UP, KRAS_SIGNALING_DN, EGFR_UP.V1_UP, KEGG_CELL_CYCLE, HALLMARK_APOPTOSIS and HALLMARK_G2M, and FDR corrected P-values were calculated. Differential expression analysis for compound treatments 6 and 7 at 4 h and at 24 h at concentrations of 30 µM and 100 µM in the Mel Juso cell line was performed using the R package edgeR. Each compound × time × concentration group was contrasted with a matched control. Gene-set enrichment was calculated as described above on all Hallmark pathways, and FDR correction to P-values was calculated. Figures and fgsea analysis were created with R v.4.1.0 using the R packages fgsea v.1.18.0, ggplot2 v.3.3.6, cowplot v.1.1.1 and ggpubr v.0.4.0.
Mouse studies
Xenograft studies were performed at Novartis and strictly adhered to Novartis BioMedical Research Animal Care and Use Committee protocols and regulations. Studies were approved by the Cantonal Veterinary Office of Basel Stadt, Switzerland, and are in strict adherence to the Swiss Federal Animal Welfare Act and Ordinance. Mice were kept under optimal hygiene conditions in individually ventilated cages under 12 h:12 h dark:light conditions and controlled temperature (20–24 °C) and humidity (45–65%) with access to sterilized food and water ad libitum. Subcutaneous tumours were induced by injecting cells in HBSS containing 50% BD matrigel in the flank of female athymic Crl:NU(NCr)-Foxn1nu-homozygous nude mice (Charles River; MugMel2_shNT, MugMel2_shNRAS, MugMel2_shSHOC2, 6 × 106) or female and male C.B-Igh-1b/IcrTac-Prkdcscid mice (Taconic; IPC298_shNT, IPC298_shNRAS, IPC298_shSHOC2 (169009) and IPC298_shSHOC2 (169012); 10 × 106). To induce shRNA KD, animals received either daily doxycycline treatments (Sigma Aldrich; 25 mg kg−1, once a day, orally) or were fed ad libitum with doxycycline-spiked food (Sigma Aldrich; 625 mg per kg diet). For efficacy studies, doxycycline treatment was started when the average tumour size reached approximately 200–300 mm3. Tumour size was measured twice weekly by calipers, and body weights were recorded twice weekly. Tumour size was calculated using the formula (length × width2) × π/6 mm3. Data are presented as mean ± s.e.m. Animals were killed at the relevant time point, after 2–3 weeks of treatment. Tumour samples for biomarker analyses were collected, snap frozen in liquid nitrogen and stored frozen at −80 °C until further processing.
RAS protein production
DNA sequence coding for the G domains of different RAS variants (amino acids 1–169) were inserted in a plasmid that allowed protein expression in Escherichia coli under control of the T7 promoter. For biotinylated proteins, BirA ligase was coexpressed with the gene of interest in the presence of 1 mM biotin. Expression was induced by 0.5 mM IPTG and performed at 18 °C overnight. Bacterial pellets were resuspended in lysis buffer (20 mM Tris pH 8.0, 500 mM NaCl, 5 mM imidazole, 2 mM TCEP, 10% glycerol, Complete protease inhibitor (Roche) and 40 U ml−1 Turbonuclease (Sigma)). Cells were lysed with high-pressure homogenizer (Avestin Emulsiflex C3) and the lysate was clarified by ultracentrifugation at 40,000g for 40 min at 4 °C. Clarified lysate was loaded on Ni-Sepharose HP (Cytiva). Beads were washed and the protein was eluted with a 5–200 mM imidazole gradient in IMAC buffer (20 mM Tris pH 8.0, 500 mM NaCl, 2 mM TCEP and 10% glycerol). The fusion-tag of the eluted protein was cleaved overnight at 4 °C by either His-tagged HRV 3C or TEV protease. Cleaved protein was reloaded onto the Ni-NTA resin (reverse-IMAC step) and flow-through containing the target protein was collected. The protein was concentrated with a 10 kDa MWCO ultrafiltration system (Millipore) and loaded on a HiLoad 16/600 Superdex 75 pg size-exclusion column pre-equilibrated with SEC buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 5 mM MgCl2 and 2 mM TCEP). Fractions containing the protein of interested were pooled, concentrated with a 10 kDa molecular-weight cut-off ultrafiltration system and snap frozen. Nucleotide exchange was performed by incubating the RAS proteins during 1 h at room temperature with a 24-fold molar excess of nucleotide (either GMPPNP (Jena Bioscience) or GDP (Sigma)) in the presence of 25 mM EDTA. The mixture buffer was then exchanged using a PD-10 column (Cytiva) against nucleotide loading buffer (40 mM Tris pH 8.0, 200 mM (NH4)2SO4 and 0.1 mM ZnCl2). A fresh 24-fold molar excess of nucleotide was again added. Then, 10 U of shrimp alkaline phosphatase (New England Biolabs) was added to samples containing GMP=PNP. After incubation for 1 h at 4 °C, MgCl2 was added to a concentration of 30 mM.
SHOC2-FL and SHOC2 amino acids 80–582 protein production
DNA sequences coding for different SHOC2 variants (for avi-tagged, also SHOC2 amino acids 93–582; for His-tagged, SHOC2 FL and G290A) were inserted in a plasmid that enabled generation of recombinant baculovirus according to the Bac-to-Bac system from Invitrogen. After virus addition to SF21 insect cells, expression was performed during 96 h at 27 °C. For biotinylated proteins, BirA ligase was coexpressed with the gene of interest in the presence of 1 mM biotin. The cell pellet was resuspended in lysis buffer (50 mM Tris pH 8.0, 300 mM NaCl, 20 mM imidazole, 10% glycerol, 0.2% Triton X-100, Complete protease inhibitor (Roche) and 40 U ml−1 TurboNuclease (Sigma)). Cells were lysed by sonication and the lysate was clarified by ultracentrifugation at 40,000g for 40 min at 4 °C. Clarified extract was loaded on a HisTrap HP 5 ml column (Cytiva). The column was washed and the protein was eluted with a 20–300 mM imidazole gradient in IMAC buffer (50 mM Tris pH 8.0, 300 mM NaCl and 10% glycerol). Eluted protein was dialysed against dialysis buffer (50 mM Tris pH 8.0, 300 mM NaCl, 0.1 mM TCEP and 10% glycerol) and cleaved overnight at 4 °C by His-tagged HRV 3C protease. Cleaved protein was reloaded on a HisTrap column (reverse-IMAC step) and the flow-through containing the target protein was collected. The protein was concentrated with a 30 kDa MWCO ultrafiltration system (Millipore) and loaded on a Superdex 200 pg size-exclusion column (Cytiva) pre-equilibrated with SEC buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1 mM TCEP and 10% glycerol). Fractions containing the protein of interest were pooled, concentrated using an ultrafiltration system and snap frozen. For assays requiring the poly-histidine tag, protein samples were prepared omitting the HRV 3C cleavage and reverse-IMAC steps.
Protein crystallography
The sitting-drop vapour-diffusion method was used for crystallization of all SHOC2 complexes. SHOC2–NRAS Q61R co-complex crystals were obtained by mixing NRAS Q61R (1–168) and SHOC2 (80–582) in a 1:1.2 molar ratio at a 15 mg ml−1 final concentration. The protein mixture solution was mixed 1:1 with 0.2 µl of well solution (0.1 M Hepes pH 7 and 15% PEG 4000) and incubated at room temperature. Crystals were cryoprotected using the crystallization solution with the addition of 20% glycerol followed by flash-freezing directly into liquid nitrogen. The SHOC2 peptide crystals were obtained by first incubating SHOC2 80–582 and peptide in a 1:1.5 ratio at a final concentration of 14 mg ml−1 SHOC2. Next, 50 mM stock of peptide in DMSO was mixed with the protein solution in storage buffer resulting in 0.65% DMSO in the protein–ligand solution. This solution was mixed, incubated briefly and then mixed 1:1 with well solution (0.05 M Hepes pH 7.5, 35% v/v pentaerythritol propoxylate 5/4 PO/OH (PP 5/4 PO/OH) and 0.2 M KCl) and incubated at 4 °C. Crystals were cryoprotected by adding 2 µl of well solution supplemented with 20% glycerol and 325 µM peptide in 0.65% DMSO directly onto the existing drop. Crystals were then collected and flash frozen in liquid nitrogen.
Micro-seeding and soaking
To produce the maximum quantity of high-quality crystals for micro-seeding, we manually refined the original condition for production of SHOC2–peptide crystals. A gradient of pH/buffering agent versus PP 5/4 PO/OH concentration (15–40% v/v PP 5/4 PO/OH; pH range of 5.5–9 with 50 mM Bis-Tris pH 5.5, 50 mM Bis-Tris pH 6.0, 50 mM HEPES pH 6.5, 50 mM Tris-HCl pH 7.0, 50 mM HEPES pH 7.5, 50 mM Tris pH 8.0, 50 mM HEPES pH 8.5 and 50 mM Bicine pH 9.0) in a 24-well plate was set with identical protein and ligand conditions in a 2 µl drop. One condition (50 mM Tris pH 7.0, 200 mM KCl and 40% PP 5/4 PO/OH) resulted in many large crystals. To produce micro-seeds for apo SHOC2 (amino acids 80-582), we first confirmed that crystals with the new condition diffracted in the same space group by collecting two crystals without further cryoprotection and collecting data in an identical way to the original structure. Wells containing many of these crystals were collected for micro-seeding by adding 10 µl of well solution onto the 2 µl drop. After gentle mixing with a pipette, the solution was aspirated into a microtube containing a seed bead (Hampton research, HR2-320). Next, 40 μl of well solution was used to further dilute the solution in the same tube. Following the manufacturer’s instructions, the tube was vortexed for 3 min to break up the crystals. Then, 450 µl of well solution was added to further dilute the microseeds. Serial dilutions of this stock (1:10, 1:100 and 1:1,000) were produced using well solution and each dilution was used as the well solution and mixed 1:1 with a stock of SHOC2 80-582 (14 mg ml−1) in a 24-well plate. Multiple dilutions of seed stock yielded usable crystals, and the 1:1,000 was selected for collection without further cryoprotection. Data collection and molecular replacement using an identical workflow revealed an apo SHOC2 structure with one copy of SHOC2 in the asymmetric unit similar to the SHOC2–peptide structure (data not shown). Because the crystal contacts in the P212121 space group allowed more room for ligand binding at the SHOC2 binding site, we soaked solutions of (R)-5 into these SHOC2 apo crystals to obtain a suitable dataset.
NMR (protein observed)
All spectra were acquired at a temperature of 310 K using a 600-MHz Bruker Avance NEO equipped with a QCI cryo-probe. SOFAST-HMQC42 spectra of 13C methyl methionine-labelled SHOC2 (80–582) were acquired in 50 mM sodium phosphate pH 7.0 and 10% D2O with 11.1 μM DSS at a protein concentration of 35 μM. Data were processed using NMRPipe43:
$${{\rm{CSP}}}_{{\rm{Hz}}}=\sqrt{{\left(\genfrac{}{}{0ex}{}{1}{}{{\rm{H}}}_{{\rm{Hz}}}\right)}^{2}+{\left(\genfrac{}{}{0ex}{}{13}{}{{\rm{C}}}_{{\rm{Hz}}}\right)}^{2}}.$$
The dissociation constant for binding of compound 1 to SHOC2(80–582) was estimated by nonlinear least-squares fitting of the observed differences in chemical shift (Δδobs Hz) between the ligand-free form (δfree) and the ligand-bound form (δobs) at multiple ligand concentrations. The following relationship was used:
$$\Delta {\delta }_{\text{obs}}={\delta }_{\text{obs}}-{\delta }_{\text{free}},$$
$$\Delta {\delta }_{\text{obs}}=\Delta {\delta }_{\max }\frac{({P}_{{\rm{T}}}+{L}_{{\rm{T}}}+{K}_{{\rm{D}}})-\sqrt{{({P}_{{\rm{T}}}+{{L}}_{{\rm{T}}}+{K}_{{\rm{D}}})}^{2}-4{P}_{{\rm{T}}}{L}_{{\rm{T}}}}}{2{P}_{{\rm{T}}}},$$
where Δδmax is a fitted parameter that corresponds to δfree − δbound and δbound is the estimated shift (Hz) of the fully bound state, PT is the total protein concentration and LT is the total the ligand concentration.
Assignment 13C methionine methyl NMR spectra
Resonance assignments for M173 and M219 were made by mutagenesis. M173 was mutated to Ala and M219 was mutated to Ala. Isotope labelling of SHOC2 was achieved by adding 1 g l−1 l-methionine (methyl-13C) (Cambridge Isotope Laboratories, CLM-206-1) to ESF921 Delta Series methionine-deficient medium (Expression Systems, 96-200). SF21 cells were grown to a density of 8 × 106 cells per ml in ESF921 (Expression Systems, 96-001-01). These cells were then diluted to a density of 0.8 × 106 cells per ml by adding sterile ESF921Delta Series methionine-deficient medium supplemented with 1 g l−1 l-methionine (methyl-13C) and grown for 3-4 d until a density of 8 × 106 cells per ml was reached. Cells were again diluted to a density of 0.8 × 106 cells per ml using ESF921Delta Series methionine-deficient medium. On the day before infection, cells were diluted to a density of 1.0 × 106 cells per ml into a final volume of 6 l of ESF921Delta Series methionine-deficient medium supplemented with 1 g l−1 l-methionine (methyl-13C) (split between two 5-l shaker flasks) and allowed to grow to a density of 1.7 × 106 to 1.8 × 106 cells per ml (18–24 h). Cells were then infected with 10 ml P2 virus per litre of culture; protein expression then proceeded for 3 d at 27 °C. Each sample was purified as described for unlabelled SHOC2(80–582). For NMR experiments, the protein was gel filtrated into 50 mM sodium phosphate pH 7.0, 100 mM NaCl, 1 mM TCEP and 10% D2O and used at a final protein concentration of 35 µM. A SOFAST-HMQC for each mutant was then compared with the spectrum of wild-type SHOC2(80–582) to determine the assignment of each methionine.
Sedimentation velocity analytical ultracentrifugation
The sedimentation-velocity experiments were done as described previously10. In brief, samples were dialysed into analytical ultracentrifugation buffer (20 mM Bis-Tris pH 6.5, 500 mM NaCl, 0.5 mM MgCl2, 1 mM TCEP and 5 µM GMP-PNP), loaded into dual-sector centrifuge cells with 1.2-cm centre pieces and sapphire windows, and these were then loaded into an AN-50 Ti rotor and allowed to equilibrate to 20 °C for 2 h before the start of high-speed sedimentation at 42,000 rpm. Data collected at 280 nm were examined with c(s) analysis41 (SEDFIT v.5.01b). Results in the form of a c(s) plot were visualized using Gussi44.
SPR to assess affinity of low-molecular-weight ligands
C-terminally avi-tagged and biotinylated Shoc2 (80–582) and full-length Shoc2 were immobilized on a Streptavidin-coated sensorchip (Cytiva, BR-1005-31). For immobilization, 1 µg ml−1 Shoc2-avi was injected with a flow rate of 10 µl min−1 for 10 min at 22 °C chip temperature in 50 mM HEPES pH 7.5, 150 mM NaCl, 1 mM TCEP and 0.1% Tween20. Sensor-chip equilibration was carried out with immobilization buffer until a stable baseline level was achieved. After immobilization, injections of 50 µM biotin solution (50 µl min−1 for 60 s) was done to reduce unspecific binding. The running buffer was 50 mM HEPES pH 7.5, 150 mM NaCl, 1 mM TCEP, 0.1% Tween20 and 2% DMSO. Experiments were done at 22 °C using a flow rate of 50 µl min−1 (contact time 60 s and dissociation time 90 s). Compounds were assessed at 12 concentrations of a 1:2.5 serial dilution series of the compounds. The stock compound dose response was prepared by diluting compounds in 100% DMSO. From the DMSO stock dilution series, dilution was done in running buffer without DMSO (1:50) to the final concentration and injected. DMSO solvent correction was used to correct for bulk effect. A serial dilution of an in-house identified low-molecular-weight binder was included in each run to confirm sensitivity between experiments. Also, a fixed concentration of this binder was injected between test compounds to assess the stability of the signal throughout the run. All SPR analyses were run on a Biacore 8k instrument. Data were processed using Biacore insight evaluation software v.4.0.8.20368. The raw data were double referenced, that is, the response of the measuring flow cell was corrected for the response of the reference flow cell, and in a second step the response of a blank injection was subtracted. Outlier sensorgrams were removed if necessary. The sensorgrams were fitted by applying a 1:1 binding model to calculate kinetic rate constants and equilibrium dissociation constants when possible. Rmax was set at global, whereas RI was set as constant (or fitted globally if needed). Data were processed individually for each run. The generated values were used to calculate average values and standard deviations of the binding constants.
SPR to assess SHOC2–RAS protein–protein interactions
Ligand affinities were determined by SPR using a Biacore 8k device (GE Healthcare). C-terminally avi-tagged and biotinylated RAS constructs were immobilized on a Streptavidin-coated sensorchip (Cytiva, BR-1005-31). For the immobilization, 0.04 µg ml−1 RAS-avi were injected with a flow rate of 10 µl min−1 for 10 min at a 22 °C chip temperature in 50 mM HEPES pH 7.4, 150 mM NaCl, 1 mM MgCl2, 10 µM nucleotide (GMP-PNP, GDP or GTP, depending on the loading state of the RAS proteins assessed), 1 mM TCEP and 0.1% Tween20. Sensor-chip equilibration was carried out with immobilization buffer until a stable baseline level was achieved. After immobilization, one injection of 50 µM biotin solution (50 µl min−1, 60 s) was carried out to reduce unspecific binding. The running buffer was the same as the immobilization buffer. Experiments were carried out at 22 °C using a flow rate of 25 µl min−1 (contact time 60 s, dissociation time 250 s). Untagged recombinant Shoc2(80–582) or full-length Shoc2 was tested in a 1:2.5 serial dilution series with a maximum concentration of 25 µM prepared in running buffer. All SPR analyses were run on a Biacore 8k instrument. Data were processed using Biacore insight evaluation software v.4.0.8.20368. The raw data were double referenced, that is, the response of the measuring flow cell was corrected for the response of the reference flow cell, and in a second step, the response of a blank injection was subtracted. Outlier sensorgrams were removed if necessary. The sensorgrams were fitted by applying a 1:1 binding model to calculate kinetic rate constants and equilibrium dissociation constants when possible. Rmax was set at global, whereas RI was set as constant. Data were processed individually for each run. The generated values were used to calculate average values and standard deviations of the respective binding constants. In the same run, only one loading state (GDP, GMP-PNP or GTP) was assessed and the respective nucleotide was supplemented in the immobilization/running buffer.
TR-FRET assay SHOC2–NRAS Q61R
Protein–compound interactions interfering with Shoc2 binding to NRAS Q61R:GTP were detected and quantified using a sensitive TR-FRET-based competitive-binding assay with a europium-labelled anti-His antibody (Eu-aHisAb, Lance Eu W1024 anti-6× His, PerkinElmer) and a Cy5-streptavidin conjugate (Cy5-SA; GE Healthcare/Amersham, PA45001). Separate pre-incubations of N-terminally His-tagged recombinant human full-length SHOC2 (amino acids 1–582) with Eu-aHisAb, and C-terminally avi-tagged biotinylated recombinant human NRAS Q61R:GTP (amino acids 1–169) with Cy5-SA were carried out for 30 min. The test compounds were dissolved in 100% DMSO and added to the solution containing the full-length SHOC2/Eu-aHisAb by means of a TTP LabTech Mosquito or a LabCyte ECHO555 pipettor. After 60 min of incubation, the NRASQ61R:GTP/Cy5-SA solution was added using a Fritz Gyger AG CERTUS pipettor. After another incubation of 60 min, the assay plates were measured using a PHERAStar FSX (BMG Labtech, Germany) microtitre plate reader equipped with dual-wavelength detection (europium excitation at 337 nm, europium emission at 620 nm and Cy5 emission at 665 nm). The final assay concentrations were: 10 nM His–SHOC2 full length, 50 nM NRASQ61R:GTP-avi, 0.25 nM Eu-aHisAb and 12.5 nM Cy5-SA. The final compound concentration for high-throughput screening at a single concentration was 31.25 µM and up to 300 µM during compound concentration, resulting in a final concentration of 0.9% DMSO for the high-throughput screen and 3% for compound characterization, respectively. The preincubation and the following measurements were conducted in assay buffer comprising 50 mM HEPES, 50 mM NaCl, 1 mM MgCl2, 50 µM GTP, 0.5 mM TCEP, 0.005% (w/v) Tween-20 and 0.005% (w/v) BSA, adjusted to pH 7.0. All solutions were handled at room temperature (22 °C).
The EC50 value was calculated from the plot of the percentage of protein saturation versus the test-compound concentration by a logistics fit according to:
$$y={\rm{A}}2+({\rm{A}}1-{\rm{A}}2)/(1+{(x/{{\rm{EC}}}_{50})}^{p}),$$
where y is the percentage saturation value at the test compound concentration, x. A1 is the lowest saturation value (0%) and A2 is the maximum saturation value (100%). The exponent, p, is the Hill coefficient.
TR-FRET assay to assess PPI disruption
To confirm the specificity of the disruption between SHOC2 and RAS, TR-FRET using a resistant mutant, SHOC2(G290A), was set up. The protocol followed the same process as described above, using His-tagged full-length SHOC2(G290A) and NRAS(Q61R):GTP. The assay buffer was identical to the one used in the SHOC2–NRAS(Q61R) TR-FRET assay. Final concentrations were 5 nM SHOC2(G290A), 20 nM NRAS(Q61R), 0.25 nM Eu-aHisab, 5 nM Cy5-SA and 3% DMSO. Because the TR-FRET for the SHOC2 mutant used protein concentrations below the KD determined by SPR (Fig. 4c), the assay remained sensitive for the assessment of displacement despite the potentially higher affinity of the mutant SHOC2 protein than RAS. This was also demonstrated by displacement by untagged wild-type SHOC2, resulting in a similar IC50 in both the TR-FRETs with wild-type SHOC2 and SHOC2(G290A) (Fig. 4d).
Melting-temperature evaluation
SHOC2 was diluted in a final volume of 10 μl of 1 μM final concentration in reaction buffer containing 50 mM HEPES pH7.5, 1 mM TCEP, 150 mM NaCl and 10× Sypro Orange (Invitrogen, stock 5000×). Triplicate wells were assessed for each protein. After a 30-min incubation, DSF measurements were carried out using an Opus qPCR thermal cycler (BioRad), with a ramping of 0.5 °C min−1. Evaluation of the melting temperatures was done using BioRad analysis software.
mRNA display selection
Two independent mRNA display selections were performed using an internal mRNA library encoding for 10-mer to 14-mer thioether cyclized peptides45,46,47. The coding region of the peptide library was designed as follows. The 5′ initiator AUG codon, encoding for the N-terminal chloroacetylated amino acid, was followed by 8–12 fully randomized positions comprising of trimer oligonucleotide mixtures. The trimer oligonucleotide pool included one distinct codon for every amino acid except for Met and Cys. The degenerate region was followed by a TGT encoding for the C-terminal Cys, enabling cyclization. At the 3′ end, there was a sequence encoding for the fixed spacer peptide sequence (GSGGSG) followed by an amber stop codon (TAG). The in vitro translation system was reprogrammed through Flexizyme-mediated genetic-code reprogramming, as previously described46,47. In brief, N-chloroacetyl l-Phe was used in place of the initiator methionine. Applying this reprogrammed translation system to the mRNA template afforded a 10-to-14-amino acid thioether-linked macrocyclic peptide library with each peptide containing a C-terminal Gly–Ser linker. Starting with an initial round containing less than 1013 unique cyclic peptides, SHOC2-specific binders were enriched using the leucine-rich repeat domain of SHOC2(93–582) with a biotinylated C-terminal Avi-tag immobilized onto streptavidin-conjugated magnetic beads. Nonspecific binders were removed using the streptavidin-conjugated magnetic beads only, and the binding stringency was increased in latter rounds through longer incubation times and/or wash steps. Two parallel selections cycles were performed with either low (150 mM NaCl) or high (300 mM NaCl) salt concentrations. In this way, the samples were subjected to buffer exchange by small desalting columns after in vitro translation and reverse transcription. All selection rounds were submitted for NGS to obtain the enriched peptide sequences. Following analysis, about ten different sequences were picked for chemical synthesis, but peptide 4 was found only in the panning with the higher salt concentration (300 mM NaCl) and showed a rather low enrichment of about 2% of all sequences, with a maximum in round 6 before applying the harsher washing procedure.
Generation of stable NRAS(Q61K)–SHOC2 NanoBiT cells
The HEK 293T cells used to generate the SHOC2–NRAS(Q61K)mut NanoBiT were obtained from ATCC or DMSZ and are part of the Novartis Cancer Cell Line Encyclopedia (CCLE), validated as previously described48. Cells were cultivated in DMEM growth medium (Gibco, DMEM high-glucose 4.5 g l−1, 1-26F01-I) supplemented with 1% stable l-glutamine (BioConcept, 5-10K50-H), 1% sodium pyruvate (BioConcept, 5-60F00-H) and 10% heat-inactivated FCS (Corning, 35-015-CF). The cells were stably transfected and monoclonal populations of cells were isolated by performing a standard limiting dilution procedure for the following SmBiT/LgBiT combinations: SHOC2–SmBiT full length/LgBiT–NRAS(Q61K)mut full length, SmBiT fused to LgBiT with a 128-amino acid linker in between as a control, to assess off target of the tested compounds. LgBiT–NRAS(Q61K) expression levels were determined by western blot using an anti-LgBiT antibody (Promega, N7100). SHOC2–SmBiT levels were determined by western blot using an anti-SHOC2 antibody (Cell Signaling Technology, 53600).
Compound dilution
Compound stock solution was prepared in DMSO/H2O (90:10) by the compound hub at Novartis Pharma AG. Compounds were tested with a seven-point or eight-point serial dilution 1:3 with a starting concentration of 30 µM.
SHOC2–NRAS(Q61K)mut NanoBiT assay
To assess protein–protein interactions in cells using NanoBiT technology49, HEK 293T SHOC2–SmBiT–LgBIT–NRAS(Q61K), HEK 293T SmBiT–128-amino acid–LgBiT cells were cultured in DMEM growth media. Cells were passaged by centrifugation in the respective culture media and split into fresh media at a ratio of 1:20 twice week. Before the NanoBiT cell-based immunoassay, 20,000 cells, were seeded in 100 µl of complete growth medium supplemented with 0.1% FCS per well of a 96-well plate (Thermo Fisher 96-well, flat-bottom microplate, 136102). Plates were incubated at 37 °C and 5% CO2 for 2 h. After incubation, cells were treated using an HP300 dispenser with increasing concentrations of compound, seven-point serial dilution 1:3 starting from 30 µM to 40 nM as the lowest concentration for 4 h. After incubation, the medium was replaced by 60 µl of Opti-MEM w/o Phenol red (Gibco, 11058), adding 15 µl of NanoGlo Live substrate (Promega, N2012) diluted 1:60 in Opti-MEM w/o Phenol red. Plates were mixed on an orbital shaker for 5 min and incubated for an extra 10 min at room temperature. Detection of luminescence signal intensity was performed using a Pherastar FSX (BMG Labtech). Absolute qualified AC50 values were analysed using the standard Novartis in-house assay data analysis software (Helios software application, Novartis Institutes for BioMedical Research, unpublished) using the methods described50,51,52,53,54. With regard to the experiment with the SHOC2(G290A) mutant, HEK 293T cells were plated in 96-well plates at a density of 20,000 cells per well and incubated overnight at 37 °C and 5% CO2. In the following days, cells were transfected with SHOC2(Gly290)–Ala-SmBiT and LgBiT–NRAS(Q61K) and incubated again overnight at 37 °C and 5% CO2. Cells were treated using an HP300 dispenser with increasing concentrations of compound, seven-point serial dilution 1:3 starting from 10 µM to 40 nM as the lowest concentration for 4 h. After incubation, 25 µl of NanoGlo Live substrate (Promega, N2012) diluted 1:60 in Opti-MEM w/o Phenol red was added. Plates were mixed on an orbital shaker for 5 min and incubated for an extra 10 min at room temperature. Detection of luminescence signal intensity was performed using a Pherastar FSX (BMG Labtech).
Chemical synthesis
General
Unless otherwise noted, all commercially available chemicals and solvents were used as received. Solvents for peptide synthesis and purifications were of high-performance liquid chromatography (HPLC) grade or higher. Chemicals were purchased from Sigma Aldrich, VWR or eMolecules.
Analytical ultra-high-performance liquid chromotography–mass spectrometry (UPLC-MS) was performed using a Waters ACQUITY UPLC. Method A: column, CORTECS C18, 2.7 µm, 2.1 × 50 mm at 80 °C; eluent A, water + 0.05% HCOOH + 3.75 mM NH4OAc; eluent B, isopropanol + 0.05% HCOOH; gradient, 5–50% B in 1.4 min, 50–98% in 0.3 min; flow, 1.0 ml min−1. Method B: Waters UPLC Acquity; column, BEH C18, 1.7 µm, 2.1 × 100 mm at 80 °C; eluent A, water + 0.05% HCOOH + 3.75 mM NH4OAc; eluent B, isopropanol + 0.05% HCOOH; gradient, 5–60% B in 8.4 min, 60–98% in 1.0 min; flow, 0.4 ml min−1. Method C: system coupled to a Xevo G2-S QTof MS (ESI); column, Acquity UPLC CSH C18, 1.7 µm, 2.1 × 100 mm at 80 °C; flow, 0.5 ml min−1; eluent A, water + 0.05% TFA; eluent B, MeCN + 0.04% TFA; gradient, hold 5% for 0.2 min, 5–98% B in 9.2 min. Peaks were detected using a DAD-UV chromatogram TIC at 210 nm and compounds were identified by electro-spray mass spectra (± mode).
Compounds were purified on a normal-phase flash chromatography system (Teledyne ISCO CombiFlash Rf+ Lumen) using prepacked silica-gel cartridges with the indicated mobile phase; or on reverse phase preparative HPLC acidic method: instrument, Büchi Pure 835; column, Waters XBridge C18, OBD Prep column, 30 × 250 mm; flow, 40 ml min−1; or water XSelect CSH C18 OBD, 30 × 100 mm; flow, 30 ml min−1, 5 µm particle size; detector, UV (220/254/280/320 nM) + ELSD; mobile phase, 0.1% aqueous TFA + acetonitrile; gradient, 2 min at a lower indicated proportion of acetonitrile, 15 min from lower to higher indicated proportion of acetonitrile, 3.5 min to higher indicated proportion of acetonitrile; column at room temperature; or on reverse-phase preparative HPLC non-acidic method: instrument, Waters AutoPurification system; column, Waters XBridge C18, OBD Prep Column, 30 × 150 mm; flow, 50 ml min−1, 5 µm particle size; detector, Waters 2489 UV/visible detector; mobile phase, 0.08% aqueous ammonium hydrogenocarbonate + acetonitrile; gradient, 1 min at 5% acetonitrile, 11 min 5–80% acetonitrile, 14 min at 100% acetonitrile; column at room temperature.
Preparative chiral HPLC method: instrument, Shimadzu4; column, Daicel Chiralpak IC, 250 × 4.6 mm, 5 µm particle size; detector, UV (220/280 nM); mobile phase, isocratic heptane-dichloromethane-EtOH (75:15:10) + 0.05% (25% aqueous NH3); flow, 1 ml min−1; column temperature, 25 °C.
Preparative chiral supercritical fluid chromatography (SFC) method: instrument, ACCQPrep SFC; column, Daicel Chiralpak AD column, 250 × 30 mm, 5 µm particle size; detector, UV (210/254 nM); mobile phase; eluent A, sc-CO2, eluent B, EtOH + 0.05% (25% aqueous NH3); isocratic elution, 45% B; flow, 95 ml min−1; column temperature, 40 °C; pressure, 100 bar.
Analytical chiral SFC method: instrument, Shimadzu LC-30-ADSF; column, Daicel as indicated, 100 ×4.6 mm, 5 µm particle size; detector, diode array; mobile phase, eluent A, sc-CO2; eluent B, as indicated; flow, 3 ml min−1; column temperature, 40 °C; pressure, 122.5 bar.
1H and 13C NMR were measured on various Bruker Avance spectrometers at room temperature, and data were reported as follows: chemical shift (ppm) from an internal standard, multiplicity (s, singlet; d, doublet; dd, double doublet; ddd, double doublet of doublet; dt, doublet of triplet; m, multiplet; and brs, broad singlet), coupling constant J (Hz), and integration.
The LC-HRMS analyses were performed by dissolving the sample with a concentration of 1 mg ml−1 in ACN:H2O (7:3). LC/ESI-MS data were recorded using an Orbitrap Lumos (Thermo Fisher Scientific) mass spectrometer equipped with an electrospray ionization source and coupled to a Thermo Ultimate 3000 liquid chromatograph equipped with a diode array detector. The chromatography separation was achieved with an Acquity UPLC BEH C18 1.7 µm, 1.0 × 50 mm column. The accurate mass was obtained by averaging 6 scans at a mass resolution of around 70,000 or 120,000 (full width at half-maximum). The mass accuracy of the system has been found to be better than 2 ppm. The chromatography was performed at 150 µl min−1 flow rate with a gradient of 5–100% B acetonitrile with 0.05% formic acid in 9 min. The mobile phase A was water with 0.04% formic acid.
Abbreviations
DIAD, diisopropyl azodicarboxylate; DIC, N,N′-diisopropylcarbodiimide; DIPEA, N,N-diisopropylethylamine; DMF, dimethyl formamide; DODT, 2,2′-(ethylendioxy)diethanthiol; EtOAc, ethyl acetate; LiHMDS, lithium hexamethyldisilazide; MeCN, acetonitrile; NMP, N-methylpyrrolidone; Pd-C, palladium on activated charcoal; Pd(dbpf)Cl2, 1,1′-bis-(di-tert-butylphosphino)-ferrocene palladium(II) dichloride; Pd(dppf)Cl2, 1,1′-bis-(diphenylphosphino)-ferrocene palladium(II) dichloride; Rt, retention time; TFA, trifluoroacetic acid; THF, tetrahydrofuran; TIS, triisopropylsilane.
Synthesis of diastereomeric mixture 1 and compounds 2 and 3
To a solution of tert-butyl (S)-(5-bromo-2,3-dihydrobenzofuran-3-yl)carbamate (prepared as described in ref. 55; 503 mg, 1.60 mmol), 4,4,4′,4′,5,5,5′,5′-octamethyl-2,2′-bi(1,3,2-dioxaborolane) (488 mg, 1.92 mmol; CAS, 73183-34-3) and potassium acetate (472 mg, 4.81 mmol) in dioxane (15 ml) under argon was added Pd(dppf)Cl2 (77 mg, 0.105 mmol). The reaction mixture was sealed and stirred for 18.5 h at 90 °C. The reaction mixture was cooled to room temperature and diluted with EtOAc (80 ml) and brine (30 ml). The organic layer was washed with brine (30 ml), dried over Na2SO4, filtered and evaporated to dryness. The crude residue was purified by normal-phase flash chromatography (24 g silica gel, cyclohexane/EtOAc 0% to 25%) to give after evaporation of the pure fractions and drying under vacuum 490 mg (83% yield) of tert-butyl (S)-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2,3-dihydrobenzofuran-3-yl)carbamate (1a, Supplementary Fig. 1a) as an off-white solid (98% pure; Rt 1.39 min; MS (ESI+, m/z) 384.2 (M + Na)+; method A).
To a solution of tert-butyl 2-(2-((3-bromobenzyl)oxy)phenyl)acetate (prepared as described in ref. 55; 281 mg, 745 μmol) in THF (2.3 ml) cooled at −78 °C was added dropwise a solution 1 M in THF of LiHMDS (1.9 ml, 1.9 mmol). The reaction mixture was stirred for 15 min at −78 °C before adding a solution of chlorotrimethylsilane (219 mg, 2.01 mmol) in THF (0.25 ml). The reaction mixture was stirred for 15 min at −78 °C before adding N-bromosuccinimide (135 mg, 0.751 mmol). The reaction mixture was allowed to slowly reach room temperature and was stirred at this temperature for 17 h. The reaction mixture was poured onto water (40 ml) and extracted twice with EtOAc (40 ml). The combined organic layers were washed with brine (20 ml) then dried over Na2SO4, filtered and evaporated to dryness. The crude residue was separated by normal-phase flash chromatography (40 g silica gel, cyclohexane/EtOAc 0% to 20%) to give after evaporation an impure intermediate that was further subjected to purification by reverse-phase preparative HPLC (XSelect, 35% to 100% MeCN in 0.1% aqueous TFA). The fractions containing the intermediate were lyophilized to give 190 mg of tert-butyl 2-bromo-2-(2-((3-bromobenzyl)oxy)phenyl)acetate (1b) as a lyophilizate (84% pure). To the lyophilizate in NMP (2.8 ml) was added 5-aminobenzo[d]oxazol-2(3H)-one (126 mg, 0.839 mmol; CAS, 14733-77-8). The reaction mixture was stirred for 21 h at 60 °C. The reaction mixture was cooled to room temperature and poured onto water (30 ml) and extracted twice with EtOAc (40 ml). The combined organic layers were washed twice with brine (30 ml), dried over Na2SO4, filtered and evaporated to dryness to afford 227 mg (34% yield) of crude tert-butyl 2-(2-((3-bromobenzyl)oxy)phenyl)-2-((2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)amino)acetate (1c) (90% pure; Rt 1.42 min; MS (ESI+, m/z) 525.1, 527.1 (Br pattern) [M + H]+; MS (ESI−, m/z) 523.1, 525.1 (Br pattern) [M-H]−; method A).
To a solution saturated with argon of compound 1c (187 mg, 320 μmol), compound 1a (178 mg, 483 μmol) and Pd(dbpf)Cl2 (19 mg, 29.2 μmol) in THF (3 ml) was added 2.4 M aqueous Cs2CO3 (0.335 ml, 804 μmol). The reaction mixture was sealed and stirred for 4.5 h at 90 °C. The reaction mixture was cooled to room temperature and diluted with EtOAc (80 ml) and brine (20 ml). The organic layer was dried over Na2SO4, filtered and evaporated to dryness. The crude residue was purified by normal-phase flash chromatography (24 g silica gel, cyclohexane/EtOAc 0% to 60%) to give after evaporation of the pure fractions and drying under vacuum, 197 mg (91% yield) of tert-butyl 2-(2-((3-((S)-3-((tert-butoxycarbonyl)amino)-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)phenyl)-2-((2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)amino)acetate (1d) as a yellowish sticky oil (100% pure; Rt 7.13 min; MS (ESI+, m/z) 680.3 [M + H]+; MS (ESI−, m/z) 678.3 [M-H]−; method B). The diastereomeric mixture (87 mg) was separated by preparative chiral HPLC to afford after evaporation of the pure fractions and drying under vacuum, 40 mg of the first eluting diastereomer 2a (100% pure; Rt 7.13 min; MS (ESI+, m/z) 680.3 [M + H]+; MS (ESI−, m/z) 678.3 [M-H]−; method B; more than 99% de; analytical chiral SFC; Chiralpak IC; 25% EtOH + 0.05% (25% aqueous NH3); Rt 3.81 min) and 39 mg of the second eluting diastereomer 3a (100% pure; Rt 7.13 min; MS (ESI+, m/z) 680.3 [M + H]+; MS (ESI−, m/z) 678.3 [M-H]−; method B; 95.4% de; analytical chiral SFC; Chiralpak IC; 25% EtOH + 0.05% (25% aqueous NH3); Rt 4.37 min).
Compound 1d (90 mg, 132 μmol) was treated with 4 M HCl (3 ml, 12 mmol) in dioxane. The reaction mixture was stirred for 6 h at room temperature. The reaction mixture was blown off with a flux of N2 and dried under vacuum to afford 74 mg (96% yield) of the title diastereomeric mixture as crude monohydrochloride salt (97% pure). The crude salt (20 mg) was purified by reverse-phase preparative HPLC (XSelect, 5% to 80% MeCN in 0.1% aqueous TFA). The pure fractions were lyophilized to give 17 mg of (R/S)-2-(2-((3-((S)-3-(amino)-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)phenyl)-2-((2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)amino)acetic acid (1, diastereomeric mixture 1:1) as a TFA salt lyophilizate (97% pure; Rt 3.25 min; MS (ESI−, m/z) 522.2 [M-H]−; method B). Each diastereomer (R)-2-(2-((3-((S)-3-(amino)-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)phenyl)-2-((2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)amino)acetic acid and (S)-2-(2-((3-((S)-3-(amino)-2,3-dihydrobenzofuran-5-yl)benzyl)oxy)phenyl)-2-((2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)amino)acetic acid of the diastereomeric mixture 1 was synthesized from the separated diastereomers 2a and 3a using the same procedure, but adapted to the scale, as described above for diastereomeric mixture 1 but without being able to attribute which displayed the configuration (S,S) or (R,S). Compound 2a (40 mg) afforded 35 mg (97% yield) of monohydrochloride salt 2 as an off-white solid (97% pure; Rt 3.24 min; MS (ESI−, m/z) 522.2 [M-H]−; method B; more than 99% de; analytical chiral SFC; Chiralpak IG; 50% EtOH + 0.05% (25% aqueous NH3); Rt 2.20 min; HRMS (m/z): [M-H]− calculated (calcd) for C30H24N3O6, 522.1665; found, 522.1672; 1H-NMR (500 MHz, DMSO) δ 11.29 ppm (d, J = 3.4 Hz, 1H), 8.71 (d, J = 13.8 Hz, 3H), 7.96 (d, J = 4.8 Hz, 1H), 7.73 (s, 1H), 7.60 (dd, J = 8.4, 2.1 Hz, 1H), 7.57–7.51 (m, 1H), 7.51–7.45 (m, 2H), 7.40 (dd, J = 7.6, 1.7 Hz, 1H), 7.28 (ddd, J = 9.0, 7.4, 1.7 Hz, 1H), 7.14 (dd, J = 8.4, 1.1 Hz, 1H), 6.99 – 6.92 (m, 2H), 6.90 (d, J = 8.6 Hz, 1H), 6.42 (d, J = 2.3 Hz, 1H), 6.30 (dd, J = 8.8, 2.5 Hz, 1H), 5.43 (s, 1H), 5.28 (s, 2H), 5.10–5.06 (m, 1H), 4.75 (dd, J = 10.9, 8.2 Hz, 1H), 4.56 (dd, J = 11.0, 3.6 Hz, 1H), 4.02 (dd, J = 172.1, 8.4 Hz, 1H), 3.72–3.66 (m, 1H) and compound 3a (39 mg) afforded 35 mg (99% yield) of monohydrochloride salt 3 as an off-white solid (96% pure; Rt 3.20 min; MS (ESI−, m/z) 522.2 [M-H]−; method B; more than 95% de; analytical chiral SFC; Chiralpak IG; 50% EtOH + 0.05% (25% aqueous NH3); Rt 4.09 min; HRMS (m/z): [M-H]− calcd for C30H24N3O6, 522.1665; found, 522.1672; 1H-NMR (500 MHz, DMSO) δ 11.32 ppm (s, 1H), 8.79 (s, 3H), 7.99 (d, J = 2.0 Hz, 1H), 7.73 (s, 1H), 7.59 (dd, J = 8.4, 2.1 Hz, 1H), 7.57–7.51 (m, 1H), 7.51–7.43 (m, 2H), 7.40 (dd, J = 7.6, 1.7 Hz, 1H), 7.28 (ddd, J = 8.9, 7.4, 1.7 Hz, 1H), 7.15 (d, J = 8.1 Hz, 1H), 7.00–6.93 (m, 2H), 6.91 (d, J = 8.6 Hz, 1H), 6.44 (d, J = 2.3 Hz, 1H), 6.31 (dd, J = 8.7, 2.3 Hz, 1H), 5.44 (s, 1H), 5.28 (s, 2H), 5.12–5.06 (m, 1H), 4.76 (dd, J = 10.9, 8.2 Hz, 1H), 4.56 (dd, J = 10.9, 3.6 Hz, 1H), 4.04 (dd, J = 163.8, 8.7 Hz, 1H), 3.73–3.65 (m, 1H).
Synthesis of peptide 4
CEM Pro Tide Rink amide resin (172 mg, 0.1 mmol, 0.58 mmol g−1) was suspended in DMF and allowed to swell for 30 min. The peptide sequence was synthesized using a CEM LibertyBlue synthesizer. The Fmoc-protecting group was removed by treatment with 5% pyrrolidine in DMF at 90 °C for 2 min. Amide couplings were performed using Fmoc-protected amino acids (4 eq., 0.2 M in DMF), DIC (8 eq., 1 M in DMF) and Oxyma Pure (8 eq, 1 M in DMF) at 90 °C for 4 min. The assembled peptide chain was N-terminally capped using chloro-acetic acid (4 eq., 0.2 M in DMF) under the same conditions.
The resin was transferred into a cartridge with frit and washed sequentially with DMF and DCM. The resin was treated with TFA/water/TIS/DODT (9.25:2.5:2.5:2.5, 6 ml) for 1.5 h and then filtered. The filtrate was collected in dry ice-cooled diethyl ether/heptane (1:1, 60 ml). The suspension was centrifuged (4,500 rpm for 6 min) and the solution carefully decanted. The obtained solids were suspended in dry ice-cooled diethyl ether (60 ml) and centrifuged again. After decanting the supernatant, the crude linear peptide pellet was dried under a stream of argon. Subsequently, it was dissolved in acetonitrile/water (1:1, 25 ml) and DIPEA (0.5 ml) was added. The mixture was agitated at room temperature for 1 h, then frozen and lyophilized to afford the crude cyclic peptide Ac{14}-Phe-Lys-Asp-Trp-Tyr-Gly-Glu-Ile-Trp-Phe-Asp-Gly-Val-Cys{0}-NH2 (4) (Supplementary Fig. 1b). Peptide 4 was purified by preparative HPLC (XBridge, 5% to 80% acetonitrile in 0.08% aqueous NH4HCO3). This afforded peptide 4 in a salt-free form (39.8 mg, more than 99% purity, method C; 22% yield). HRMS (m/z): [M + 2H]2+ calcd. for C88H112N18O22S 902.3954, found 902.3942 (Supplementary Fig. 1c).
Synthesis of racemic compound 5, (S)-5 and (R)-5
To a suspension of methyl 2-(2-hydroxyphenyl)acetate (500 mg, 3 mmol; CAS, 22446-37-3), (5-bromo-2-chlorophenyl)methanol (816 mg, 3.61 mmol; CAS, 149965-40-2) and polystyrene polymer supported triphenylphosphine (2.45 g, 3.63 mmol) in anhydrous THF (20 ml) under argon was added DIAD (730 mg, 3.61 mmol; CAS, 2446-83-5). The reaction mixture was stirred for 20.5 h at room temperature. The reaction mixture was filtered over Celite. The filtrate was diluted with EtOAc (80 ml) and was washed twice with brine (20 ml) then was dried over Na2SO4, filtered and evaporated to dryness. The crude residue was purified by normal-phase flash chromatography (40 g silica gel, cyclohexane/EtOAc 0% to 25%) to give after evaporation of the pure fractions and drying under vacuum, 976 mg (88% yield) of methyl 2-(2-((5-bromo-2-chlorobenzyl)oxy)phenyl)acetate (5a; Supplementary Fig. 1d) as an off-white solid (100% pure; Rt 1.50 min; MS (ESI+, m/z) 369.0, 371.0 (BrCl pattern) [M + H]+; method A). To a solution of compound 5a (976 mg, 2.64 mmol) in anhydrous THF (8 ml) cooled at −78 °C was added dropwise a solution 1 M in THF of LiHMDS (6.9 ml, 6.9 mmol). The reaction mixture was stirred for 15 min at −78 °C before adding a solution of chlorotrimethylsilane (774.5 mg, 7.13 mmol) in THF (2 ml). The reaction mixture was stirred for 30 min at −78 °C before adding N-bromosuccinimide (500 mg, 2.78 mmol). The reaction mixture was allowed to slowly reach room temperature and was stirred 16.5 h at this temperature. The reaction mixture was poured onto water (80 ml) and extracted twice with EtOAc (80 ml). The combined organic layers were washed with brine (60 ml) then dried over Na2SO4, filtered and evaporated to dryness. The crude residue was separated by flash chromatography (40 g silica gel, cyclohexane/dichloromethane 0% to 25%) to give after evaporation of the fractions and drying under vacuum, 1.02 g (74% yield) of methyl 2-bromo-2-(2-((5-bromo-2-chlorobenzyl)oxy)phenyl)acetate (5b) as a yellowish sticky oil (86% pure; Rt 1.56 min; method A). To compound 5b (200 mg, 446 µmol) under argon in NMP (2.5 ml) was added 5-aminobenzo[d]oxazol-2(3H)-one (135 mg, 899 µmol; CAS, 14733-77-8). The reaction mixture was stirred for 1.5 h at 80 °C. The reaction mixture was cooled to room temperature and poured onto water (70 ml) and extracted with EtOAc (60 ml). The organic layer was washed twice with brine (20 ml), dried over Na2SO4, filtered and evaporated to dryness. The crude residue was purified by reverse-phase preparative HPLC (XBridge, 40% to 100% MeCN in 0.1% aqueous TFA). The pure fractions were evaporated to dryness to give 171 mg (71% yield) of methyl 2-(2-((5-bromo-2-chlorobenzyl)oxy)phenyl)-2-((2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)amino)acetate (5c) as an off-white foam (96% pure; Rt 1.27 min; MS (ESI+, m/z) 516.9, 518.8, 520.9 (BrCl pattern) [M + H]+; MS (ESI−, m/z) 515.0, 516.9, 518.9 (BrCl pattern) [M-H]−; method A). To a solution saturated with argon of compound 5c (171 mg, 317 μmol), phenylboronic acid (45 mg, 358 μmol; CAST, 98-80-6), Pd(dbpf)Cl2 (30 mg, 46 μmol) in THF (4 ml) was added 2.4 M aqueous Cs2CO3 (0.33 ml, 792 μmol). The reaction mixture was sealed and stirred for 3 h at 90 °C. The reaction mixture was cooled to room temperature and diluted with EtOAc (80 ml) and brine (20 ml). The organic layer was dried over Na2SO4, filtered and evaporated to dryness. The crude residue was separated by normal-phase flash chromatography (12 g silica gel, cyclohexane/EtOAc 0% to 50%) to give after evaporation of the pure fractions and drying under vacuum, 125 mg (65% yield) of methyl 2-(2-((4-chloro-[1,1′-biphenyl]-3-yl)methoxy)phenyl)-2-((2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)amino)acetate (5d) as an off-white foam (85% pure; Rt 1.41 min; MS (ESI+, m/z) 515.3, 517.3 (Cl pattern) [M + H]+; MS (ESI−, m/z) 513.3, 515.2 (Cl pattern) [M-H]−; method A). To a solution of compound 5d (125 mg, 206 μmol) in dioxane (3.5 ml) was added 1 M aqueous LiOH (0.7 ml, 700 μmol). The reaction mixture was sealed and stirred for 1.25 h at 40 °C. The reaction mixture was cooled to room temperature and 2 M aqueous HCl (66.1 μl, 132 μmol) was added. The reaction mixture was cooled to room temperature and quenched with water (30 ml). The solution was adjusted around pH 2 with 2 M aqueous HCl and extracted twice with EtOAc (40 ml). The combined organic layers were dried over Na2SO4, filtered and evaporated to dryness. The crude residue was purified by reverse-phase preparative HPLC (XSelect, 40% to 100% MeCN in 0.1% aqueous TFA). The pure fractions were concentrated and the suspension was cooled for 1 h at 4 °C. The precipitate was filtered and washed with water and then dried under vacuum to give 83 mg (79%) of (rac)-2-(2-((4-chloro-[1,1′-biphenyl]-3-yl)methoxy)phenyl)-2-((2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)amino)acetic acid (5) as a white solid (98% pure; Rt 6.32 min; MS (ESI+, m/z) 501.0, 502.9 (Cl pattern) [M + H]+; MS (ESI−, m/z) 499.1, 501.1 (Cl pattern) [M-H]−; method B; HRMS (m/z): [M + H]+ calcd for C28H22ClN2O5, 501.1217; found, 501.1215; 1H-NMR (600 MHz,d6-DMSO) δ 12.75 ppm (brs, 1H), 11.21 (s, 1H), 8.00 (d, J = 2.3 Hz, 1H), 7.68 (dd, J = 8.3, 2.3 Hz, 1H), 7.65–7.58 (m, 3H), 7.45–7.33 (m, 4H), 7.31 (td, J = 7.8, 1.7 Hz, 1H), 7.18 (d, J = 8.2 Hz, 1H), 6.99 (t, J = 7.5 Hz, 1H), 6.86 (d, J = 8.6 Hz, 1H), 6.40 (t, J = 2.1 Hz, 1H), 6.28 (dt, J = 8.7, 2.0 Hz, 1H), 5.44 (d, J = 2.2 Hz, 1H), 5.38–5.30 (m, 2H); 13C-NMR (151 MHz, d6-DMSO) δ 172.85 ppm, 155.60, 154.89, 144.42, 139.21, 138.60, 135.20, 134.87, 131.10, 130.86, 129.82, 129.17, 128.96, 128.08, 127.81, 127.61, 127.25, 126.97, 126.58, 121.17, 112.58, 109.49, 105.37, 94.73, 67.06, 54.23. The racemic mixture 5 (73 mg) was separated by preparative chiral SFC to afford after evaporation of the respective separated fractions and drying under vacuum followed by reverse-phase preparative HPLC (XSelect, 30% to 100% MeCN in 0.1% aqueous TFA), 23 mg as an off-white solid of title compound enantiomer (S)-5 (96% pure; more than 99.9% ee; analytical chiral SFC; Chiralpak IG 100 × 4.6 mm 5 µm 35% (iPrOH + 0.05% aqueous 25% NH3); Rt 3.41 min), the absolute configurations of which were assigned post hoc by co-crystallization (PDB: 9BTP) and 24 mg as an off-white solid of title compound enantiomer (R)-5 (95% pure; 99.6% ee: analytical chiral SFC; Chiralpak IG 100 × 4.6 mm 5 µm 35% (iPrOH + 0.05% aqueous 25% NH3); Rt 4.15 min).
Synthesis of compounds 6 and 7
A solution of 4-(benzyloxy)-3-nitrobenzaldehyde (490 mg, 1.91 mmol; CAS, 22955-07-3), (S)-2-methylpropane-2-sulfinamide (277 mg, 2.29 mmol; CAS, 343338-28-3), copper(II) sulfate (851 mg, 5.33 mmol) in dichloromethane (4.800 ml) was sealed and stirred for 5 d at 50 °C. The reaction mixture was cooled to room temperature and filtered over Celite. The remaining solid was washed with dichloromethane (50 ml). The filtrate was evaporated to dryness and the crude residue was purified by normal-phase flash chromatography (40 g silica gel, dichloromethane/EtOAc 0% to 20%) to give after evaporation of the pure fractions and drying under vacuum, 603 mg (88% yield) of (S,E)-N-(4-(benzyloxy)-3-nitrobenzylidene)-2-methylpropane-2-sulfinamide (6a; Supplementary Fig. 1e) as an off-white solid (100% pure; Rt 1.24 min; MS (ESI+, m/z) 361.1 [M + H]+; method A; HRMS (m/z): [M + H]+ calcd for C18H21N2O4S, 361.1222; found, 361.1217; 1H-NMR (500 MHz, d6-DMSO) δ 8.56 ppm (s, 1H), 8.47 (d, J = 2.1 Hz, 1H), 8.22 (dd, J = 8.8, 2.2 Hz, 1H), 7.61 (d, J = 8.9 Hz, 1H), 7.50–7.32 (m, 5H), 5.42 (s, 2H), 1.18 (s, 9H)). To a solution of intermediate 6a (440 mg, 1.22 mmol) in dichloromethane (8 ml) under argon was added BF3.OEt2 (346.5 mg, 2.44 mmol). The reaction mixture was stirred for 0.75 h at room temperature and then was added a solution of ((1-methoxy-2-methylprop-1-en-1-yl)oxy)trimethylsilane (425.6 mg, 2.44 mmol) in dichloromethane (4 ml). The reaction mixture was sealed and stirred for 21.5 h at room temperature. The reaction mixture was diluted with dichloromethane (40 ml) and brine (20 ml). The aqueous layer was extracted with dichloromethane (40 ml) and the combined organic layers were dried over Na2SO4, filtered and evaporated to dryness. The crude residue was purified by normal-phase flash chromatography (80 g silica gel, dichloromethane/EtOAc 0% to 40%) to give after evaporation of the pure fractions (first eluting diastereomer) and drying under vacuum, 164.3 mg (29% yield) of methyl (R)-3-(4-(benzyloxy)-3-nitrophenyl)-3-(((S)-tert-butylsulfinyl)amino)-2,2-dimethylpropanoate (6b) as an oil (100% pure; Rt 1.18 min; MS (ESI+, m/z) 463.4 [M + H]+; MS (ESI−, m/z) 461.3 [M − H]−; method A; HRMS (m/z): [M + H]+ calcd for C23H31N2O6S, 463.1903; found, 463.1897; 1H-NMR (500 MHz, d6-DMSO) δ 8.03 ppm (d, J = 2.3 Hz, 1H), 7.70 (dd, J = 8.8, 2.3 Hz, 1H), 7.49–7.38 (m, 5H), 7.38–7.32 (m, 1H), 5.57 (d, J = 11.1 Hz, 1H), 5.31 (s, 2H), 4.56 (d, J = 11.1 Hz, 1H), 3.59 (s, 3H), 1.09 (s, 9H), 1.08 (s, 3H), 0.96 (s, 3H). From the second eluting diastereomer methyl (S)-3-(4-(benzyloxy)-3-nitrophenyl)-3-(((S)-tert-butylsulfinyl)amino)-2,2-dimethylpropanoate (7a) was recovered 187.5 mg (33% yield) as an oil (94% pure; Rt 1.18 min; MS (ESI+, m/z) 463.5 [M + H]+; MS (ESI−, m/z) 461.3 [M − H]-; method A; HRMS (m/z): [M + H]+ calcd for C23H31N2O6S, 463.1903; found, 463.1897; 1H-NMR (500 MHz, d6-DMSO) δ 7.84 ppm (d, J = 2.2 Hz, 1H), 7.58 (dd, J = 8.8, 2.3 Hz, 1H), 7.48–7.38 (m, 5H), 7.38–7.31 (m, 1H), 5.47 (d, J = 8.0 Hz, 1H), 5.28 (s, 2H), 4.59 (d, J = 8.1 Hz, 1H), 3.63 (s, 3H), 1.14 (s, 3H), 1.05 (s, 3H), 1.01 (s, 9H) and was used to assess by small-molecule X-ray the configuration of the newly formed stereocentre, to synthesize, by analogy to its diastereomer compound 6c, 26.8 mg (yield 21%) of methyl (S)-3-(((S)-tert-butylsulfinyl)amino)-2,2-dimethyl-3-(2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)propanoate (7b) as an off-white solid (99% pure; Rt 3.68 min; MS (ESI+, m/z) 369.1 [M + H]+; MS (ESI-, m/z) 367.2 [M-H]−; method B; HRMS (m/z): [M + H]+ calcd for C17H25N2O5S, 369.1484; found, 369.1479; 1H-NMR (500 MHz, d6-DMSO) δ 11.59 ppm (s, 1H), 7.20 (d, J = 8.2 Hz, 1H), 7.02–6.95 (m, 2H), 5.37 (d, J = 7.5 Hz, 1H), 4.53 (d, J = 7.5 Hz, 1H), 3.63 (s, 3H), 1.15 (s, 3H), 1.04 (s, 3H), 1.00 (s, 9H); 13C-NMR (126 MHz, d6-DMSO) δ 175.96, 154.42, 142.44, 135.10, 129.84, 122.11, 109.61, 108.55, 65.41, 55.38, 51.89, 47.67, 22.48, 22.14, 20.97 (CCDC-2353396 shows S,S-configuration; www.ccdc.cam.ac.uk/structures). A solution of compound 6b (164.0 mg, 355 μmol) in THF (3.600 ml) and EtOAc (1.800 ml) was flushed with N2 and Pd-C (50 mg, 10% weight, 47.0 μmol) was added. The reaction mixture was flushed with H2 and stirred for 2 h at room temperature under 1.1 bar H2. The reaction mixture was filtered over Celite and the catalyst was washed with EtOAc (25 ml) and THF (10 ml). The filtrate was evaporated and dried to give 128 mg of crude intermediate as an off-white solid (90% pure; Rt 0.62 min; MS (ESI+, m/z) 343.1 [M + H]+; MS (ESI−, m/z) 341.2 [M − H]−; method A). The solid was taken in dichloromethane (3.400 ml) and triethylamine (103.3 mg, 1.020 mmol) was added. The solution was cooled in an ice bath and diphosgene (40.4 mg, 204.1 μmol) was added, then the reaction mixture was stirred for 0.5 h at 0 °C and 75 min at room temperature. The reaction mixture was cooled in an ice bath and quenched with saturated aqueous NaHCO3 (1 ml) then diluted with dichloromethane (40 ml) and brine (20 ml). The aqueous layer was extracted with dichloromethane (30 ml) and the combined organic layers were washed with brine (20 ml), dried over Na2SO4, filtered and evaporated to dryness. The crude residue was purified by normal-phase flash chromatography (24 g silica gel, dichloromethane/MeOH 0% to 5%) to give after evaporation of the pure fractions and drying under vacuum, 45.4 mg (33% yield) of methyl (R)-3-(((S)-tert-butylsulfinyl)amino)-2,2-dimethyl-3-(2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)propanoate (6c) as a film (97% pure; Rt 3.69 min; MS (ESI+, m/z) 368.9 [M + H]+; MS (ESI−, m/z) 367.1 [M-H]−; method B; HRMS (m/z): [M + H]+ calcd for C17H25N2O5S, 369.1484; found, 369.1479); 1H-NMR (500 MHz, d6-DMSO) δ 11.69 ppm (s, 1H), 7.2–7.16 (m, 2 H), 7.06 (dd, J = 8.4, 1.8 Hz, 1H), 5.48 (d, J = 10.9 Hz, 1H), 4.51 (d, J = 10.9 Hz, 1H), 3.58 (s, 3H), 1.12–1.04 (m, 12H), 0.95 (s, 3H); 13C-NMR (126 MHz, d6-DMSO) δ 175.95, 154.60, 142.47, 135.54, 129.71, 122.50, 109.97, 108.34, 66.67, 55.90, 51.68, 47.81, 23.39, 22.57, 19.92. To a solution of compound 6c (20.1 mg, 54.6 μmol) in dichloromethane (0.350 ml) was added 4 M HCl in dioxane (27.3 μl, 109 μmol). The reaction mixture was sealed and stirred for 1.25 h at room temperature. The reaction mixture was blown off with a flux of N2 and dried under vacuum. To the residue under argon was added a solution of 2-(3-iodophenyl)acetic acid (17.2 mg, 65.5 μmol; CAS, 1878-69-9) in DMA (0.550 ml) preliminarily treated with triethylamine (19.3 mg, 26.6 μl, 3.5 eq, 191 μmol) and TPTU (22.7 mg, 1.4 eq, 76.4 μmol) for 3 min at room temperature. The reaction mixture was stirred for 40 min at room temperature. The reaction mixture was diluted with EtOAc (50 ml) and washed with brine (4 × 25 ml), dried over Na2SO4, filtered and evaporated to dryness. The crude residue was purified by normal-phase flash chromatography (12 g silica gel, dichloromethane/EtOAc 0% to 60%) to give after evaporation of the pure fractions and drying under vacuum, 16.8 mg (59% yield) of methyl (R)-3-(2-(3-iodophenyl)acetamido)-2,2-dimethyl-3-(2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)propanoate (6d) as a film (98% pure; Rt 1.00 min; MS (ESI+, m/z) 509.0 [M + H]+; MS (ESI−, m/z) 507.1 [M-H]−; method A). To a solution of compound 6d (16.8 mg, 33.1 μmol) in dioxane (0.330 ml) was added 1 M aqueous LiOH (132 μl, 132 μmol). The reaction mixture was sealed and stirred for 5.5 h at 50 °C. The reaction mixture was cooled at room temperature and 2 M aqueous HCl (66.1 μl, 132 μmol) was added. The reaction mixture was blown off with N2 flux and the crude residue was purified by reverse-phase preparative HPLC (XBridge, 5% to 100% MeCN in 0.1% aqueous TFA). The pure fractions were concentrated and lyophilized to give 10.1 mg (61%) of (R)-3-(2-(3-iodophenyl)acetamido)-2,2-dimethyl-3-(2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)propanoic acid (6) as a white lyophilizate (98% pure; Rt 4.36 min; MS (ESI+, m/z) 495.0 [M + H]+; MS (ESI−, m/z) 493.1 [M – H]−; method B; 94% ee; analytical chiral SFC; Chiralpak IH; 30% MeOH + 0.05% (25% aqueous NH3); Rt 2.34 min; HRMS (m/z): [M + H]+ calcd for C20H20IN2O5, 495.0417; found, 495.0411; 1H-NMR (500 MHz,d6-DMSO) δ 12.49 ppm (brs, 1H), 11.66 (s, 1H), 8.48 (d, J = 9.8 Hz, 1H), 7.61 (t, J = 1.7 Hz, 1H), 7.56 (ddd, J = 7.8, 1.8, 1.1 Hz, 1H), 7.27–7.17 (m, 2H), 7.08 (t, J = 7.8 Hz, 1H), 7.02–6.95 (m, 2H), 5.24 (d, J = 9.8 Hz, 1H), 3.57–3.45 (m, 2H), 1.06 (s, 3H), 1.01 (s, 3H); 13C-NMR (151 MHz, d6-DMSO) δ 177.12, 169.14, 154.54, 142.30, 139.01, 137.57, 135.60, 135.00, 130.35, 129.88, 128.41, 121.68, 109.33, 108.63, 94.57, 57.62, 46.29, 41.35, 22.27, 21.17. (S)-3-(2-(3-iodophenyl)acetamido)-2,2-dimethyl-3-(2-oxo-2,3-dihydrobenzo[d]oxazol-5-yl)propanoic acid (7) was obtained as a white lyophilizate (100%; Rt 4.30 min; MS (ESI+, m/z) 495.1 [M + H]+; MS (ESI−, m/z) 493.0 [M – H]−; method B; more than 99)% ee; analytical chiral SFC; Chiralpak IH; 30% MeOH + 0.05% (25% aqueous NH3); Rt 2.03 min; HRMS (m/z): [M + H]+ calcd for C20H20IN2O5, 495.0417; found, 495.0410; 1H-NMR (500 MHz,d6-DMSO) δ 12.46 ppm (brs, 1H), 11.64 (s, 1H), 8.48 (d, J = 9.5 Hz, 1H), 7.61 (t, J = 1.8 Hz, 1H), 7.56 (d, J = 7.7 Hz, 1H), 7.27–7.17 (m, 2H), 7.08 (t, J = 7.7 Hz, 1H), 7.02–6.95 (m, 2H), 5.24 (d, J = 9.7 Hz, 1H), 3.55–3.46 (m, 2H), 1.06 (s, 3H), 1.02 (s, 3H); 13C-NMR (151 MHz, d6-DMSO) δ 177.13, 169.12, 154.54, 142.30, 139.00, 137.57, 135.63, 135.00, 130.33, 129.88, 128.40, 121.68, 109.33, 108.60, 94.55, 57.66, 46.26, 41.36, 22.30, 21.23) by analogy to the conversion of intermediate 6c to compound 6 starting from intermediate 7b (overall yield 57%).
Ligandability analysis (Supplementary Fig. 2)
a, Protein–protein interaction interface between SHOC2 (green cartoons) and NRAS (orange). b, A single ligandable site (pink surface) in SHOC2 is located at the SHOC2–RAS interface. c, Peptide 4 (blue surface) identified by an unbiased screen binds at the same site at the PPI interface. d, Low-molecular-weight compound (R)-5 (yellow surface) overlaps in the same binding site. e, Atomistic overlap of peptide 4 (blue sticks) and compound (R)-5 (yellow sticks) in their binding site. Although the peptide is larger and extends further towards the N-terminal part of SHOC2, it overlaps with the low-molecular-weight compound interface. Key contacts are preserved between the peptide and the low-molecular-weight compounds. f, Highlight of the key carbolic acid of Asp11 of peptide 4 and compound (R)-5 exhibiting a similar salt bridge to R223 and hydrogen bond to Q269. g, Highlight of key greasy interactions between Phe10 and Val13 of peptide 4 and the chloro-biphenyl group of compound (R)-5 extending hydrophobic contacts with the alkyl parts of the side chains of E311, R288, N265, T242 and D244. The 3D structure of apo SHOC2 does not exhibit conventional buried cavities that could host a small molecule. Although relatively large pockets can be found at the SHOC2–PP1CA and SHOC2–MRAS interaction interfaces12, the surface of apo SHOC2 is rather flat. However, there is a hydrophobic patch on the concave side of SHOC2, at the PPI with RAS. This patch has a limited buried surface of 236 Å3, an average hydrophobicity of 34% and an overall ligandability estimated to be moderate17 (PDB code: 7YTG). The main hydrophobic residues of the pocket are the side chains of Met219 and Thr242 and the alkyl chains of Asn265, Arg288, Glu311 and Asn313; and the backbone of residues Leu220, Leu243, Leu266, Leu289, Gly290 and Leu312. Polar residues lining the pocket are the side-chain terminal groups of Ser221, Arg223, Asp244, Asp267, Gln269, Arg288, Arg292, Glu311 and Asp313. Key residues shaping the binding site are long and flexible side chains that are free to move (three Arg and one Glu). Seven charged amino acids are defining the electrostatics and polarity of the binding site. Altogether, the limited ‘buriedness’ and hydrophobicity of this binding site, combined with the flexibility of the key amino acid side chains forming it, and the numerous formal charges in this pocket, make it particularly challenging for the development of potent low-molecular-weight binders. No other ligandable cavity can be characterized in the existing crystal structures of SHOC2, making it an arduous target for the development of inhibitors.
Statistics and reproducibility
All experiments were repeated three or more times independently, unless otherwise specified in the figure legends, with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.