The former UK prime minster Margaret Thatcher famously managed to run the country on just four hours of sleep per night. She embraced this as part of her personal brand and identity. In later life, the ‘Iron Lady’ was diagnosed with Alzheimer’s disease.
Scientists have known about a link between poor sleep and an increased risk of dementia for decades. Maiken Nedergaard, a neuroscientist at the University of Rochester in New York, says that people who report six hours or less of sleep a night are more likely to develop dementia later. “Sleep disturbances very often precede the first sign of dementia by many years,” she says.
Understanding the biological processes that underpin this connection is crucial to producing new drugs for dementia — a disease that someone in the world develops every three seconds, according to the non-profit organization Alzheimer’s Disease International. But acquiring that knowledge has proved to be a tricky and surprisingly controversial task. For the past decade or so, there was something of a consensus around a theory that emerged from a paper1 that Nedergaard and her colleagues wrote in 2013. They were the first to put forward the idea that during sleep, the brain flushes out proteins that are linked to neurodegenerative diseases — in particular tau and amyloid-β. The brain does this, they say, through its glymphatic system, which includes cerebrospinal fluid and interstitial fluid.
Nature Outlook: Sleep
But in the past year, this theory has been brought into question. Nick Franks, a biophysicist at Imperial College London, and his colleagues reported in Nature Neuroscience that brain clearance is instead reduced during sleep2. These data, he argues, mean that researchers need to go back to the drawing board to explain why good sleep reduces a person’s risk of dementia.
Amid this challenge to the prevailing brain-clearance theory, scientists have exchanged harsh criticisms, with some accusing others of personal attacks and attempts to upend an entire field of research. Tensions are running high.
A pharmaceutical awakening
The parties to this fracas agree on two key findings: that there is a link between poor quality sleep and an increased risk of dementia, and that there is a reduced concentration of dementia-linked proteins in the brain after a person has slept. The fierce disagreement is over why and how this occurs.
Scientists who back Nedergaard and her team say that it’s because the brain clears the proteins during sleep. Those who support Franks’s group say that they aren’t sure. But Franks has speculated that protein synthesis is reduced during sleep.
“Mechanistically, it matters, because you need to know what to target,” explains Brendan Lucey, a neurologist and sleep-medicine specialist at Washington University in St. Louis’s School of Medicine in Missouri. Drug makers need to know whether they should be looking for ways to generally improve sleep for people at risk of Alzheimer’s disease, or whether they should focus their efforts instead on molecules that can manipulate the brain’s protein-clearance process.
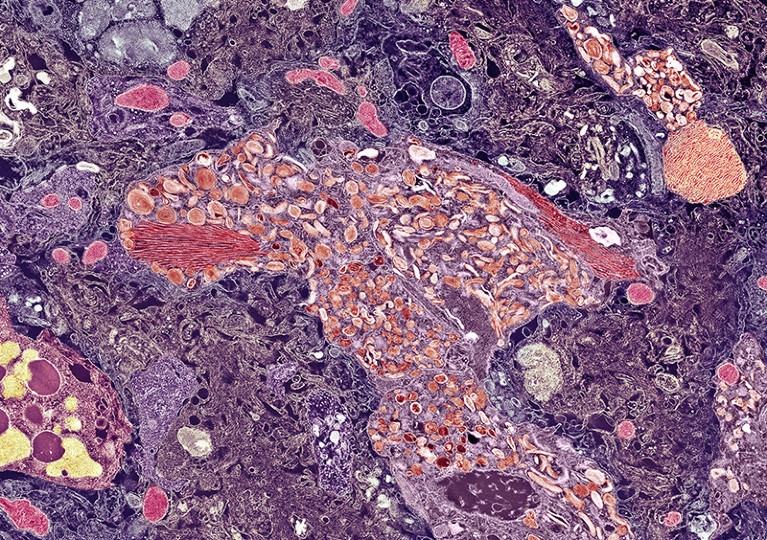
A plaque of amyloid-β (centre) in the brain of a person with Alzheimer’s disease.Credit: THOMAS DEERINCK, NCMIR/SPL
In the absence of a clear answer, both avenues are being explored. “We’re trying to figure out whether we can manipulate sleep in a way that is protective,” says David Holtzman, a colleague of Lucey and the director of the Knight Alzheimer Disease Research Center at Washington University in St. Louis, who is researching potential pharmaceutical therapies for neurodegenerative diseases. He points out that one of the most common classes of insomnia drug, which includes zolpidem (Ambien), work by enhancing the effects of the neurotransmitter GABA, thereby suppressing neural electric signalling in the brain. “The problem,” says Holtzman, “is that those are probably not something that you could take safely forever, especially in a middle-aged or older person because they cause memory problems.” And the last thing anyone worried about dementia needs is to take a drug that could potentially worsen their memory.
A nightmarish argument
In their 2013 paper, Nedergaard and her colleagues used imaging techniques to track the diffusion of tetramethylammonium ions through mouse brains. They showed that mice who were either anaesthetized or allowed to sleep naturally had a 60% higher rate of brain clearance than did those who were kept awake. This led the team to conclude that sleep increases the rate at which problematic molecules are removed from the brain, thus reducing the risk of dementia.
She has since co-authored a flurry of other papers, most recently in January3, that support this explanation. In this latest paper, Nedergaard and her co-authors looked at whether zolpidem had an effect on brain clearance. Their data showed that the drug reduced glymphatic flow, potentially reducing the number of proteins that could be removed from the brain. That could explain why the drug affects memory negatively, and gives credence to the idea that increasing sleep by any means might not lower the risk of dementia — use zolpidem for long enough, Nedergaard says, and any gains from having had more sleep could well be wiped out by the negative effects. “The risk for the long term is that you would accelerate the amyloid-β deposition.”
Franks, by contrast, used a new method to get his tracing material into mouse brains. Previous investigations injected material into the cisterna magna, an area outside the brain that supplies cerebrospinal fluid. These experiments, therefore, measure the influx of material into the brain, and assume that increased influx means that more fluid must also be flushed out. Franks’s team instead injected material directly into mice brains and measured whether it dissipated to other parts of the organ.
His results showed that the tracing materials did not disperse during sleep. In fact, the concentration of the dye was higher during both anaesthesia and sleep. This, says Franks, would suggest that sleep actually reduces clearance.

Neuroscientist Maiken Nedergaard (left) and biophysicist Nick Franks.Credit: Adam Fenster, University of Rochester; Meilin Sanchez
“What we’ve shown, to our satisfaction at least, is that the consensus that sleep clears everything from the brain more quickly is wrong, or at least questionable,” says Franks.
This has been met with frustration from Nedergaard. “If you want to disrupt a whole field, you need to use the same technical method. That’s a must if you’re disputing already established facts from many different groups. You can’t come in with completely different techniques,” she says. “It’s from someone who doesn’t know the field well.”
Franks rejects this critique, and says that it’s perfectly legitimate to use innovative techniques in science and that if new ways of looking at a phenomenon disagree with the older methods, that’s scientifically interesting in its own right.
The disagreement has been unusually intense. Nedergaard asked Franks, and other researchers, to a meeting in New York in December 2024 to discuss his findings. “We invited Franks’s team,” says Nedergaard. “And basically, everybody told them that their paper is wrong.”
Despite anticipating such an argument, Franks admits to being taken aback. “I felt like I was being invited to be ambushed and humiliated,” he says. “It wasn’t a meeting to really discuss whether we were right. It was political and it was about public relations.”
Nedergaard, meanwhile, is keen to stress that the controversy transcends a disagreement between just her and Franks. “There’s a whole bunch of papers showing that sleep in humans facilitates brain clearance, so it’s not just our story or our hypothesis,” she says.
Nedergaard has since written to the editors of Nature Neuroscience to explain why she thinks that Franks’s paper shouldn’t have been published. One of her main concerns is that Franks might have injected too much material into the brain, causing “brain-wide impacts that alter neural activity”. She also says that the paper fails to assess whether brain damage or inflammation were caused by the procedures, which could have skewed the results.
Franks rejects this criticism. “This comment completely ignores the critical parameter of the rate of injection,” he says. And factoring that into the analysis, he asserts, shows that “damage here is unlikely”.
This back and forth might sound intense, but there is a lot at stake — most importantly, scientists’ foundational understanding of the pathology of dementia. And this knowledge ultimately guides the treatment of neurodegenerative diseases, says Holtzman.
Nedergaard is right about the mass of papers that back up the brain-clearance theory. And she’s also right that, so far, Franks and his team have published only one paper on the subject. But there are others who say that Franks’s data might complement their own findings. Steven Proulx, a biomedical engineer at the University of Bern in Switzerland who studies fluid flow in the central nervous system, is one of them.
More from Nature Outlooks
Proulx and his team’s experimental design was similar to Nedergaard’s — they did not inject tracers directly into the brain, but instead into the cerebrospinal fluid4. “Most of the tracers that you inject into the cerebrospinal fluid don’t go into the brain. They mostly wash out towards the lymphatic system,” he says. “We went back to Nedergaard’s original 2013 paper and realized that she didn’t consider this other pathway for the tracers, which might mean that she has potentially misinterpreted some of her data.” Nedergaard rejects this critique. Her work is valid, she says, because she’s interested in following the tracers that do enter the brain.
The dream of a consensus
Sorting out the different sides of this argument is difficult, not least because of the intricacies of sleep. “It’s complicated because sleep affects a lot of systems in the body,” says Lucey. “To say that it’s just clearance alone is overly simplistic.” Lack of sleep influences the immune system and can cause inflammation — both of which are associated with vascular and metabolic problems that Lucey says could lead to dementia.
“The causes of sleep disturbance are legion, and the effects of sleep disturbance are many, Lucey says. “I think that’s what results in the complexity.”
Another factor that makes this disagreement difficult to resolve is that studying sleep, especially in mice, is hard to do in a controlled manner. For starters, just mounting sensors and devices on the animals can alter their behaviour. Another issue is that researchers have often fallen back on anaesthesia as a proxy for sleep, but that has its limitations. “Every anaesthetic has its own characteristic — some increase glymphatic flow, and some decrease it,” Nedergaard says. Therefore, she argues, “anaesthesia as a model is outdated”.
Nedergaard thinks that she has worked out ways to finesse the experiments to get as close to real sleep as possible. “We spent years developing this fibre optic where we implant little fibres in the brain three weeks before we actually study the mice,” she explains. “These fibres are mounted in a hook, so the mice do not have to carry them on the head.” That detail is important, she says, because “if you have anything more than three or four grams on a mouse’s head, it cannot lift it and then, of course they can’t sleep. They get very upset.” In her latest paper3, she says, “we have mice that sleep completely normally”.
More than 55 million people around the world already live with dementia and that number will only rise as life expectancy continues to increase globally. That’s why the scientific arguments and debates posed by Franks and his colleagues are so important to address. Clarifying the mechanism that links sleep and dementia will be crucial to devising the best lines of attack to counter a debilitating disease that is on the rise.




