Subjects
The following mouse lines (6–8 weeks old, male and female) were used for experiments: C57BL/6 J mice (Jackson Laboratory, 000664), NTS-Cre (Jackson Laboratory, 017525, strain code: Ntstm1(cre)Mgmj), GAD2-Cre (Jackson Laboratory, 010802, strain code: Gad2tm2(cre)Zjh/J), Ai14 (Jackson Laboratory, 007914, strain code: B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J), Ntsflox (Jackson Laboratory, 036262, strain code: B6;FVB-Ntsem1Evdr/J). Mice were housed on a 12 h:12 h light cycle (lights on at 07:00) and a room temperature of 22–25 °C and 55% humidity. All procedures complied with the animal care standards set forth by the National Institutes of Health and were approved by University of California Berkeley’s Administrative Panel on Laboratory Animal Care.
Diet protocols
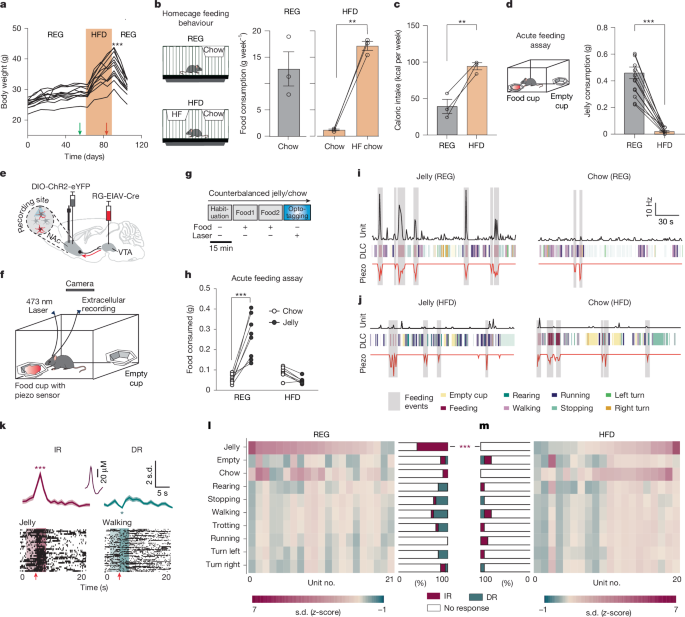
Mice subjected to a regular diet (REG) had ad libitum access to standard chow (5053 PicoLab Rodent Diet 20, Lab Diet) in their home cage. Mice subjected to a high-fat diet (HFD) mice had ad libitum access to both standard chow and 60% fat chow (Research Diets D12492) for a minimum of 4 weeks prior to experiments. The body weight of all mice was assessed at least once per week.
Stereotaxic surgeries
Surgeries were performed under general ketamine–dexmedetomidine anaesthesia using a stereotaxic instrument (Kopf Instruments, Model 1900). For retrograde tracing, mice were injected unilaterally with fluorescent retrobeads (100 nl; LumaFluor) or cholera toxin b subunit (400 nl; Fisher Scientific) into the nucleus accumbens (NAc) lateral shell (NAcLat; bregma: 1.0 mm, lateral: 1.9 mm, ventral: −4.3 mm) or ventral tegmental area (VTA; bregma: −3.3 mm, lateral: 0.4 mm, ventral: −4.5 mm) using a 1 μl Hamilton syringe (Hamilton).
The AAVs used in this study were from the Wilson laboratory (pENN.AAV.hSyn.Cre.WPRE.hGH; ~10¹³ infectious units per ml, prepared by AddGene), the Deisseroth laboratory (AAV5-EF1α–DIO-hChR2(H134R)-eYFP; AAV5-EF1α-DIO-mCherry; AAV5-hSyn-hChR2(H134R)-eYFP; AAV5-Ef1α-DIO-eYFP; AAV5-hSyn-eYFP; ~1012 infectious units per ml, prepared by the University of North Carolina Vector Core Facility), from the Boyden laboratory (AAV5-Syn-ChrimsonR-tdTomato; AAV5-CaMKII-ArchT-GFP; ~1012 infectious units per ml), from the Tian laboratory; ntsLight1.1: The NTS sensor gene was cloned into a hSyn promoter and WPRE enhancer containing SV40 poly (A) signal sequence. The vector was packaged into an adeno-associated virus serotype 9 (AAV9-ntsLight1.1-WPRE-SV40p(A), physical titre 6.45 × 1014 vg ml−1, produced by the Viral Core at the University of California Davis); ntsLight2.0: The NTS sensor gene was cloned into a hSyn promoter and WPRE enhancer containing SV40 poly (A) signal sequence. The vector was packaged into an AAV serotype 9 (AAV9-ntsLight2.0-WPRE-SV40p(A), physical titre 2.19 × 1016 vg ml−1, produced by the Viral Core at the University of California Davis; virus was diluted 5× with saline before injection), from the Földy laboratory (AAV-NTS-OE: the NTS precursor gene was cloned into a short CAG promoter and WPRE enhancer containing SV40 vector with two lox sequences on each side of the gene to make NTS expression Cre-dependent). The vector was packaged into an AAV serotype 9 (ssAAV-9/2-shortCAG-dlox-mNts(rev)-dlox-WPRE-SV40p(A), ~1013 infectious units per ml; produced by the Viral Vector Facility of the University of Zurich) and from the Lim laboratory (University of California San Diego, RG-EIAV-Cre).
For AAV injections, 300–500 nl of concentrated AAV solution was injected into the NAcLat (same coordinates as above) or VTA (same coordinates as above) using a syringe pump (Harvard Apparatus) at 150 nl min−1. The injection needle was withdrawn 5 min after the end of the infusion. Experiments were performed 6–24 weeks (for AAVs), 7 days (for retrobeads) or 2 days (for CTB–Alexa Fluor 647) after stereotaxic injections.
For in vivo electrophysiology, animals were implanted unilaterally above the NAcLat (bregma: 1.0 mm, lateral: 1.9 mm, ventral: −3.5 mm) with a custom-built driveable optoelectrode (optrode), which consisted of eight tetrodes (12-μm polyimide-coated nickel-chrome wire protected by silica tubing) glued to a 200-μm optical fibre using epoxy. The tetrodes protruded from the tip of the optical fibre by ~0.5 mm. Wire tips were cut flat, and gold plated to reduce impedance to ~200 kΩ at 1 kHz. A small screw fixed to the skull served as a ground electrode. Data collection began <1 week after the optrode implantations.
For in vivo optogenetics, animals received unilateral (for ChR2) implantations of a chronically implanted optical fibre (200 μm, NA = 0.37; Newdoon) dorsal to the VTA (bregma: −3.3 mm, lateral: 0.4 mm, ventral: −3.9 mm) or dorsal to the NAcLat (bregma: 1.0 mm, lateral: 1.9 mm, ventral: −3.6 mm). For ArchT experiments, optical fibres were implanted bilaterally and were angled (10°) above the VTA (bregma: −3.3 mm, lateral: ±1.2 mm, ventral: −4.4 mm). One layer of adhesive cement (C&B Metabond; Parkell) was followed by acrylic cement (Dental cement) to secure the optical fibre to the skull. The incision was closed with a suture and tissue adhesive (Vetbond; 3 M). The animals were kept on a heating pad until they recovered from anaesthesia. Atipamezole was injected intraperitoneally to reverse the sedative effects of dexmedetomidine.
For in vivo opto-pharmacology, animals were chronically implanted with a cannula (PlasticsOne, 33 G 4.6 mm) above the VTA (bregma: −3.3 mm, lateral: 0.4 mm, ventral: −3.9 mm). Opto-infusion experiments were done >1 week after cannula implantations. Injection sites and optical fibre placements were confirmed in all animals by preparing coronal sections (50 or 100 µm) of injection and implantation sites. Although optical fibre placements varied slightly from mouse to mouse, behavioural data from all mice were included in the study.
For in vivo fibre photometry experiments using ntsLight2.0, mice were chronically implanted with an optical fibre (400 μm, NA = 0.37; Newdoon) in the VTA (bregma: −3.3 mm, lateral: 0.4 mm, ventral: −4.5 mm) and above the NAcLat (bregma: 1.0 mm, lateral: 1.9 mm, ventral: −3.6 mm).
Anatomical nomenclature
Nucleus accumbens
The NAc, a key component of the ventral striatum, is traditionally divided into shell and core subregions, which are both anatomically and functionally distinct61. In this study and in previous studies26,62,63,64, we describe an additional ventral striatal subregion, termed NAc lateral shell, which is located laterally to the NAc core. We realize that the terminology ‘NAc lateral shell’ may be misleading since it may suggest that the NAc lateral shell is part of the NAc shell, even though these regions are anatomically and functionally different. Nevertheless, we use ‘NAc lateral shell’ (NAcLat) as it refers to an anatomical region that is defined in the The Mouse Brain in Stereotaxic Coordinates65 (bregma: 1.34 mm to 0.74 mm).
Our findings that optogenetic stimulation of the NAcLat→VTA pathway specifically increases the consumption of hedonic foods without affecting standard chow intake suggest that the role of NAc medial shell inputs to the VTA in hedonic feeding behaviour warrants further investigation. However, optogenetic stimulation of NAc medial shell terminals in the VTA predominantly inhibits dopamine neurons and induces a general state of behavioural suppression, which is not specific to either reward- or aversion-related behaviours26. This complexity would make interpreting the effects of optogenetic stimulation on feeding behaviour within the NAc medial shell pathway more challenging.
Ventral tegmental area
We defined the lateral VTA as the medio-dorsal and lateral parabrachial pigmented nucleus and the medial lemniscus region adjacent to the substantia nigra. Please note that the definition of medial versus lateral VTA is largely based on the anatomical location of projection-defined VTA dopamine neurons. It is not strictly based only on the medio-lateral axis, but also incorporates the dorso-ventral axis63.
Electrophysiology
Ex vivo electrophysiology
Mice were deeply anaesthetized with pentobarbital (200 mg kg−1, intraperitoneal injection; Vortech). Coronal brain slices containing the NAcLat or VTA (200 μm) were prepared after intracardial perfusion with ice-cold ACSF containing (in mM) 50 sucrose, 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 0.1 CaCl2, 6.174 MgCl2, 2.96 kynurenic acid (NAcLat slices only) and 2.5 glucose (oxygenated with 95% O2/5% CO2). After 60–90 min of recovery, slices were transferred to a recording chamber and perfused continuously at 2–4 ml min−1 with oxygenated ACSF, containing (in mM) 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2.5 glucose, 22.5 sucrose, 2.058 MgCl2 and 2 CaCl2 at ~30 °C. Cells were visualized with a 40× water-immersion objective on an upright fluorescent microscope (BX51WI; Olympus) equipped with infrared-differential interference contrast video microscopy and epifluorescence (Olympus). For whole-cell current clamp recordings, patch pipettes (3.8–4.4 MΩ) were pulled from borosilicate glass (G150TF-4; Warner Instruments) and filled with internal solution, which consisted of (in mM) 135 potassium gluconate, 5 KCl, 10 HEPES, 0.1 EGTA, 2 MgCl2, 2 MgATP, 0.2 NaGTP, pH 7.35 (290–300 mOsm). For perforated-patch recordings, patch pipettes were first filled with internal solution, as described above, and then back filled with internal solution containing 100 µg ml−1 gramicidin. Electrophysiological recordings were made at 32 °C using a MultiClamp700B amplifier and acquired using a Digidata 1440 A digitizer, sampled at 10 kHz, and filtered at 2 kHz. All data acquisition was performed using pCLAMP software (Molecular Devices, version 10.5). Channelrhodopsin-2 was stimulated by flashing 473 nm light through the light path of the microscope using an ultrahigh-powered light-emitting diode (LED) powered by an LED driver (Prizmatix) under computer control. A dual lamp house adapter (Olympus) was used to switch between fluorescence lamp and LED light source. The light intensity of the LED was not changed during the experiments and the whole slice was illuminated (5 mW mm−2). Series resistance (15–25 MΩ) and input resistance were monitored online. Data were analysed offline using Clampfit (Molecular Devices, version 10.5). For recordings of spontaneous action potential firing, cells were held in current clamp mode and no current injections were made. Spontaneous firing was recorded for at least 3 s before and 5 s after light stimulation (20 Hz, 3 ms light pulses, 5 mW mm−2). For pharmacological experiments, we recorded baseline responses and drugs were bath applied for 5–10 min (1 µM SR142948A (Tocris), 1 µM neurotensin (Sigma Aldrich)). To determine the neurochemical identity of retrobead-labelled neurons (that is, TH-immunopositive or TH-immunonegative), neurons were filled with 0.1% neurobiotin (Vector), which was included in the internal solution, during patch-clamp recordings, then fixed in 4% paraformaldehyde (PFA) and 24 h later immunostained for tyrosine hydroxylase. The dopaminergic phenotype was confirmed in experiments shown in Fig. 4j–m. Neurobiotin was not included in experiments shown in Fig. 3a–f and Extended Data Fig. 9a–f. A more detailed description on the neurochemical identity of retrogradely labelled neurons in the VTA can be found in Lammel et al.64.
In vivo electrophysiology
Mice with optrode implants were attached to a fibreoptic cable that was connected to a 473 nm DPSS laser diode (Laserglow) through an FC/PC adapter. Laser output was controlled using a Master-8 pulse stimulator (A.M.P.I.). Power output was tested using a digital power meter (Thorlabs) and was checked before and after each experimental animal; output during light stimulation was estimated to be 3–5 mW mm−2 at the targeted tissue 200 μm from the fibre tip. Neural signals were recorded using a Digital Lynx 4SX system with an HS-18-MM headstage pre-amplifier (Neuralynx) with a Millmax connector. Recorded signals were filtered between 0.6 and 6 kHz and sampled at 32 kHz. Spikes were sorted offline using SpikeSort3D 2.5.4 (Neuralynx) software. To identify opto-tagged cells, the optrode was advanced ~40 µm per day, and a brief light screening (30 light pulses, 5 ms width at 1 Hz) was performed to detect light-responsive cells. If activity was detected, the mice proceeded to the behavioural assessment. If no activity was found, the optrode was advanced another ~40 µm, and the mice were re-tested for light-induced cell activity the following day. After behavioural assessment, the optrode was moved ~80 µm to minimize the likelihood of recording the same cells on subsequent days, and new opto-tagged cells were screened. Food consumption experiments while recording the neural signals, consisted of 3 behavioural trials (habituation, jelly, chow, 15 min each, order of food counterbalanced, 45 min total), and an opto-tagging trial at the end of the behavioural session where light stimulation was given at 2 Hz for 2 min. At the end of the opto-tagging stage, the optrode was moved ventrally for ~40 μm until active units were detected. The final recording location was verified using histology after the electrolytic lesions (12 μA, 30 s). ChR2-tagged neurons were identified by delivering 473 nm (0.8 mW mm−2, 1–5 ms pulses) of light at 2 Hz frequency for 2–3 min. A 2-ms bin with the highest number of spikes in the interval (0, +100 ms) around the laser pulse was identified. To test if the identified strongest response to light was higher than chance, we shuffled all the spike times in the same (0, +100 ms) interval 10,000 times and counted the highest number of spikes in a 2-ms bin for each iteration. If the number of spikes in the 2 ms bin from the real data exceeded the 99.9th percentile value of the distribution of number of spikes in the most active 2-ms bin for the shuffled data, we classified the cell as light-responsive. Response latency was defined as the average response time in the most active 2 ms bin, and only units with response latency <8 ms were classified as opto-tagged. Examples of the opto-tagging procedure in REG and HFD mice are shown in Extended Data Fig. 1i–r.
Piezo-based analysis of feeding
For detection of feeding events, a piezo-based sensor was placed under the food cup. Cells were included in the analysis only if piezo sensor activity was detected. To obtain time-locked events, activation of the sensor was transmitted as a TTL signal to the Neuralynx recording system via an Arduino Uno board. Time spent feeding included the sum of events in which the sensor was activated at least twice and lasted until there was an interval larger than 6 s between the sensor activations. Piezo-detected events were confirmed by randomly inspecting 5 frames where the behaviour was determined as feeding in the analysis.
Video-based analysis of behavioural motifs
A video-based offline tracking was performed via DeepLabCut66. Specifically, DeepLabCut.py (version 2.0.7) was used to track all points of interest. The network was trained using 20 frames from 6 randomly selected videos (containing mice of different diets and food types) for >1,000,000 iterations. Horizontal xy coordinates of the nose, head, body centre and the tail base were extracted for each frame. Only DLC coordinates of 85% accuracy and higher were used in the analysis. Deduction of behavioural motifs from the DLC obtained coordinates was done using a custom-made MATLAB code. The video start time was aligned to the Neuralynx recording system using the LED readout of the piezo sensor, and the matching TTL signal that was recorded directly to the Neuralynx data acquisition system via an Arduino Uno board. Deduction of behavioural motifs: feeding/empty motifs were defined as high proximity of the head position with either cup (<5 cm). Rearing was defined by close proximity of the body and head positions (<2 cm). Body and head orientation were determined as vectors from tail to body, and from body to head, respectively. Turning behaviour was defined as turning the head more than 15° from the body centre, which was then counted across a session67. The rest of the frames were classified according to velocity. Velocity was calculated by the distance of body position between frames, normalized to the size of the open-field chamber (in cm) and the frame rate (15 frames s−1). Velocity-based behavioural motifs were defined for in vivo electrophysiology as <1 stopping, 1–5 walking, 5–10 trotting, and >10 running (units of cm s−1). Velocity motifs were not included if the mouse was too close to the food plate (<7 cm from either plate) to avoid confusion with the food/empty plate motifs. Except for turning events, each motif occurrence was included only if it persisted for <7 frames (0.5 s). The behavioural readout and motif selection were visually verified for each experiment by randomly inspecting five frames of each motif to confirm the correct motif selection (Extended Data Fig. 1g,h).
Classification of IR and DR response types
Response types during different behavioural motifs or piezo-based feeding events were classified into non-responsive, increased response (IR), and decreased responses (DR) using the following criteria: For each unit, average time series responses were collected around event onset from each motif type. Pre-event unit firing rates (−3 to 0 s before event onset) and event rates (0 to 3 s from event onset) were analysed using the Wilcoxon signed-rank test to determine statistical significance and the direction of change. IR: pre-event rates <event rates and P < 0.05; DR: pre-event rates > event rates and P < 0.05; otherwise, a unit would be classified as non-responsive. The proportion of the classified units (for both opto-tagged and non-tagged units) was analysed in REG and HFD mice and compared with the Chi-squared test; P value was corrected using the Bonferroni correction for multiple comparisons.
NeuralynxMatlabImportExport_v6.0.0 MATLAB package (available at https://neuralynx.fh-co.com/research-software/), custom MATLAB code and GraphPad Prism (versions 9.5.1 and 10.3.1) were used for analysis of in vivo electrophysiology data.
Behavioural assays
All behavioural tests were performed during the light phase (unless otherwise specified) in a temperature (20–23.5 °C) and humidity (40–60%) controlled room that is illuminated by 8× 32 W fluorescent lights each producing 2,925 lumens. Behavioural equipment was cleaned with 70% ethanol between individual animals.
Acute feeding assay
Mice were placed in a chamber (25 cm length × 25 cm width × 25 cm height) with 2 small empty food cups fixed to the floor on opposite corners. Following a 15-min habituation period, one empty cup was replaced with a cup containing a pre-measured amount of a specific food type (that is, standard chow, high-fat chow, chocolate, peanut butter, strawberry jelly, butter, water, or butter with quinine; note on preparation of butter with quinine: 30 g of butter was microwaved for 30 s to melt. 1.4 g of quinine (Q1250), dissolved in 15 ml of distilled deionized water, was thoroughly mixed into the butter. The butter–quinine mixture was then refrigerated until solidified and subsequently brought back to room temperature for use in the behavioural assay) that was weighed while the other cup remained empty. After each 15-min trial, the food cup was weighed again and subtracted from the initial weight to determine the amount of food consumed. Mice received a sample of each food type in their home cage and were habituated to the behavioural chamber on three different days prior to behavioural testing. This procedure was performed to avoid stress, and neophobia to novel foods, a characteristic behaviour in mice68.
For in vivo optogenetic activation, mice with optical fibre implants were attached to a fibreoptic cable that was connected to a 473 nm DPSS laser diode. Optogenetic experiments consisted of 5 trials in the following order: habituation, primed-feeding, OFF, ON, OFF (15 min each; 75 min total). During the habitation trial, mice could freely explore the chamber, but no food was present. We placed a new pre-measured food cup containing a specific food type into the chamber at the beginning of each subsequent trial. The primed-feeding trial allowed us to assess the effects of diet on feeding behaviour. Additionally, because feeding behaviour was reduced in the subsequent OFF trial, it allowed us to test the effects of optogenetic stimulation when feeding behaviour was low. During the ON trial, 20 Hz (or 1 Hz, 10 Hz for experiments in Extended Data Fig. 3d), 5 ms blue light was delivered through the fibreoptic cable. There was no optogenetic stimulation during the habituation, primed-feeding and OFF trials. In experiments shown in Extended Data Figs. 3e,f and 4c,d, mice were food-deprived (FD; that is, all home cage food was removed) 24 h before the start of the experiment. Food consumption during the primed-feeding trial under FD condition was compared to baseline levels of primed-feeding measured in the same, but not FD, mice (that is, baseline measurements were performed the day before initiation of FD and consisted of habituation and primed-feeding trials (15 min each)).
For in vivo opto-pharmacology (Fig. 4f–i), mice were placed in an open-field chamber and a custom-made optical fibre was inserted into the cannula for light stimulation. Same experimental design as above, except that between trial 1 and trial 2 (that is, laser OFF and ON trials), mice were placed into a separate box where they were infused with either saline or 6 mM SR142948A (500 nl via infusion pump at a rate of 300 nl min−1). There was an additional 10 min waiting period after infusion before animals were placed back into the chamber to conduct the remaining trials.
For in vivo optogenetic inhibition (Extended Data Fig. 3o–t), mice with bilateral optical fibre implants were attached to a fibreoptic cable connected to a 593 nm DPSS laser diode. The experiment was conducted over three consecutive days. On each day, mice were placed in a behavioural chamber for habituation (15 min, no food present), and jelly feeding (15 min). On day 2, constant laser light (10 mW) was delivered through the fibreoptic cable when jelly was present. Jelly consumption was measured for each day.
Home cage feeding
Mice in home cage feeding experiments received a pre-measured amount of standard chow and 60% fat chow that was measured weekly for each cage. Food consumption was normalized to the number of mice in each cage.
Open-field test
Mice were placed in a custom open-field chamber (50 cm length × 50 cm width × 50 cm height) and their movement was recorded and analysed for 10 min using video-tracking. MouseActivity5.m (https://github.com/HanLab-OSU/MouseActivity/blob/master/MouseActivity5.m) was used to analyse open-field behaviour. To assess anxiety-related behaviour, we determined the time the animals spent in the centre of the chamber (33 cm length × 33 cm width)69. For analysis of behavioural motifs using DeepLabCut (version 2.0.7), we used a similar approach as described in ‘Video-based analysis of behavioural motifs’ to identify rearing, turning, and different velocity modalities. The behavioural readout and motif selection were visually verified for each experiment by randomly inspecting five frames of each motif (Extended Data Fig. 10h).
Real-time place preference
Mice with optogenetic implants were connected to a fibreoptic cable and placed in a custom-made three-compartment chamber. For optogenetic stimulation, the cable was connected to a 473 nm DPSS laser diode (Laserglow) through an FC/PC adapter, and laser output was controlled using a Master-8 pulse stimulator (A.M.P.I.). Power output was tested using a digital power meter (Thorlabs) and was checked before and after each experimental animal; output during light stimulation was estimated to be 3–5 mW mm−2 at the targeted tissue 200 μm from the fibre tip. One side of the chamber was designated as the initial stimulation side (phase 1) and after 10 min the stimulation side was switched to the other previously non-stimulated side of the chamber (phase 2). The middle of the chamber was a neutral area that was never paired with stimulation. At the start of each session, the mouse was placed in the middle of the chamber, and every time the mouse crossed to the stimulation side, constant laser stimulation (473 nm, 20 Hz, 5 ms pulses) was delivered until the mouse exited the stimulation area. There was no interruption between Phase 1 and Phase 2. The first stimulation side was counterbalanced across mice. The movement of the mice was recorded via a video-tracking system (Biobserve, version 3.0.1.442) and the time the mice spent in each area (stimulated, non-stimulated, neutral) was calculated.
Body temperature measurements
To measure body temperature, mice were manually restrained to minimize stress during measurement, and a rectal thermometer was inserted into the rectum to a depth of about 1 cm.
Nutritional values for different food types (all values per 1 g)
Jelly (Smucker’s Strawberry Jelly; calories: 2.5 kcal, total fat: 0 g, saturated fat: 0 g, trans fat: 0 g, cholesterol: 0 g, sodium: 0 g, total carbohydrate: 0.65 g, dietary fibre: 0 g, total sugars: 0.6 g, protein: 0 g), butter (Trader Joe’s Unsalted Butter; calories: 7.14 kcal, total fat: 0.79 g, saturated fat: 0.5 g, trans fat: 0 g, cholesterol: 0 g, sodium: 0 g, total carbohydrate: 0 g, dietary fibre: 0 g, total sugars: 0 g, protein: 0 g), peanut butter (Skippy; calories: 6.39 kcal, total fat: 0.5 g, saturated fat: 0.11 g, trans fat: 0 g, cholesterol: 0 g, sodium: 0 g, total carbohydrate: 0.25 g, dietary fibre: 0.06 g, total sugars: 0.11 g, protein: 0.22 g), chocolate (Hershey Kisses; calories: 4.88 kcal, total fat: 0.29 g, saturated fat: 0.9 g, trans fat: 0 g, cholesterol: 0 g, sodium: 0 g, total carbohydrate: 0.61 g, dietary fibre: 0.02 g, total sugars: 0.56 g, protein: 0.07 g), regular chow (PicoLab Rodent Diet 20; calories: 3.02 kcal, total fat: 0.05 g, saturated fat: 0.01 g, trans fat: 0 g, cholesterol: 0.14 g, sodium: 0 g, total carbohydrate: 0.54 g, dietary fibre: 0.04 g, total sugars: 0.03 g, protein: 0.21 g), high-fat chow (Research Diets D12492; calories: 5.24 kcal, total fat: 0.35 g, saturated fat: 0.18 g, trans fat: 0 g, cholesterol: 0 g, sodium: 0 g, total carbohydrate: 0.26 g, dietary fibre: 0.06 g, total sugars: 0.25 g, protein: 0.26 g).
Development of ntsLight1.1 and ntsLight2.0
All constructs were designed using circular polymerase extension cloning and restriction cloning. BamHI and HindIII sites were introduced via PCR for final subcloning onto pAAV-hSynapsin1 vector (Addgene). To enhance coupling between conformational changes and chromophore fluorescence, we used a cpGFP module from GCaMP6s for insertion into the human NTS1R. For screening linker variants, we generated linker libraries by first designing primers with 22 C saturated mutagenesis70 for one amino acid on each side of the linker (LSS-XI-cpGFP-XH-DQL). To screen ntsLight2.0 from ntsLight1.0, based on common activation pathway of class A GPCRs71, we generated libraries at region 5.61 and 6.33 for screening. Cloned constructs were amplified and purified with the Qiagen PCR purification kit before NEB 5α Competent Escherichia coli transformation. Competent cells were plated onto kanamycin-containing agar plate. After 24 h of growth at 37 °C, single colonies were picked into 96-well plates and grown overnight. Plasmids were purified using Wizard MagneSil Plasmid Purification System (Promega, A1630) with Opentrons OT-2 liquid handler. Top variants were sequenced by Genewiz. ntsLight1.1 was discovered after linker screening (LSS-XI-cpGFP-XH-DQL) and resulted in WI-EH. ntsLight2.0 screening resulted in I259M and G301T. To make AAV plasmids, NEB stable competent cells were transformed with pAAV plasmids. After overnight growth on an ampicillin-containing agar plate at 30 °C, a single colony was selected and sequenced. The colony with the correct sequencing result was then expanded at 30 °C in 100 ml of growth medium (2×YT), purified with a Qiagen Endo-free plasmid Maxi Kit, and sent to the UC Davis Virus Core for virus production.
ntsLight1.1 measurements in cell culture assays
Cell culture preparation
Glass-bottom 96-well plates (P96-1.5H-N, Cellvis) were coated with 0.1 mg ml−1 of poly-d-lysine (Sigma, P6407-5MG) overnight. Plates were washed with water and E18 rat hippocampal neurons (BrainBits; https://tissue.transnetyx.com/E18-Rat-Hippocampus_4; not authenticated; not tested for mycoplasma contamination) were plated in neurobasal culture media with Neurobasal Plus Meidum (Gibco, A35829-01-500mL), B27 Plus Supplement (Gibco, A3582801), Glutamax (Gibco, 35050-061) and Gentamicin Reagent (Gibco, 15710-064). Neurons were infected with AAV9-hSyn-ntsLight1.1 (see above) on DIV5 neurons and changed to new media on DIV7. Half media change was performed every two days before imaging on DIV12.
Neuronal cell titration
Neuronal cell titration was performed in 96-well plate. Prior to a titration experiment on DIV12 neurons, stock solution of 10 mM neurotensin (Phoenix Pharmaceuticals) in H2O were diluted to 333 µM (in HBSS and 0.1 mg ml−1 BSA) and distributed in all of the first wells in 96-well plates. The following wells had serial dilution in HBSS for neuronal titration. For imaging with antagonist, stock solutions of 1 mM SR142948A (Millipore Sigma) in H2O were diluted to 200 nM in imaging media distributed across an empty 96-well plate (ligand plate) in triplicate. The imaging media consisted of 1× HBSS (Fisher, 14175103) containing HEPES buffer. Neurons grown in a separate 96-well plate (imaging plate) were gently washed 3x with imaging media, and the wells were filled with an appropriate volume of imaging media for the respective experiment. For titration experiments, 50 µl of imaging media was added to each well of the assay plate. Wells were then imaged with ImageXpress MicroConfocal High-Content Imaging system at 40× (NA 0.6) with 4 regions of interest (ROI) taken per well with no overlap of the ROIs (exposure = 300 ms) with MetaXpress software (version V6.6.3.55). Next, 50 µl from the ligand plate was transferred to the imaging plate containing a doubled desired final concentration. After 5 min of incubation, the same sites were re-imaged using the same settings. Titration was done with final concentration ranging from 150 µM to 1 pM, with tenfold serial dilution each time.
Ligand specificity test and validation
Neurons were plated and cultured in a 4-chamber glass-bottom dish (35 mm, Cellvis) following the same protocol as described above. Neurons were imaged using 60× oil objective on a Leica Stellaris Confocal. The neurons were imaged in imaging buffer and 10 µl of the following ligands were applied directly to each chamber: NTS (10 µM, Phoenix Pharmaceuticals), GABA (100 µM, Tocris), dopamine (100 µM, Sigma), acetylcholine (Sigma, 100 µM), 5-HT (100 µM, Fisher), oxytocin (10 µM, Phoenix Pharmaceuticals), somatostatin (10 µM, Phoenix Pharmaceuticals), neuropeptide Y (10 µM, Phoenix Pharmaceuticals), cholecystokinin (10 µM, Phoenix Pharmaceuticals), dynorphin (10 µM, Phoenix Pharmaceuticals) and neuromedin U-25 (10 µM, Phoenix Pharmaceuticals). We observed a concentration-dependent increase in fluorescence in the presence of NTS that was attenuated by application of an NTS receptor antagonist (SR142948A) (Extended Data Fig. 6a).
Image processing and analysis
Once imaging was complete, the images were exported and analysed using a customized MATLAB script (available at: https://github.com/lintianlab). In brief, segmentation was performed on individual images and a mask highlighting the membrane of the neurons was generated. Pixel intensities were obtained from the mask-highlighted area and exported into Excel. The ΔF/F values for each well were calculated.
Brain-slice recordings using ntsLight1.1
Slice preparation and imaging
Acute coronal midbrain slices were prepared (same procedure as described in ex vivo electrophysiology), transferred to a recording chamber and perfused continuously at 2–4 ml min−1 with oxygenated ACSF. Slices were visualized under a custom-built, open source macroscope (https://github.com/Llamero/DIY_Epifluorescence_Macroscope) fitted with high power LEDs and a Teledyne Kinetix sCMOS camera. Custom drawn regions of interest were imaged at a rate of 20 Hz with a 10 ms exposure of 474 nm LED stimulation (5.2 mW mm−2) for a total of 20 s. In the middle of the recording, 1 s of 635 nm stimulation (17 mW mm−2) consisting of 5 ms pulses at 20 Hz was delivered to the slice between each camera exposure, so that none of the Chrimson stimulation light was recorded. Green light stimulation experiments were performed similarly to the red-light stimulation, with 1 s of 554 nm stimulation (8 mW mm−2) consisting of 5 ms pulses at 20 Hz. For pulse-width modulation experiments, red-light stimulation was delivered at 20 Hz with varying pulse widths. To determine dF/F in the lateral VTA, the fluorescence from an ROI drawn away from sensor and Chrimson-expressing regions was divided from the fluorescence in lateral VTA. Because photobleaching curves were not identical between different regions of the tissue, an additional baseline subtraction was performed. A window of dF/F signal around Chrimson stimulation time was removed, the remaining dF/F data was smoothed, and an estimated polynomial fit trendline was drawn through the smooth data and across the removed stimulation time window. This trendline was subtracted from the complete dF/F signal. AUC was calculated as an approximate trapezoidal integral during stimulation time.
Ex vivo validation
To examine NAcLat→VTA specific NTS release, mice were injected with ntsLight1.1 into the VTA and AAV-hSyn-ChrimsonR-tdTomato (Chrimson) into the NAcLat; a separate group of mice was infused with only ntsLight1.1 into the VTA (sensor only). Six weeks later, we recorded ntsLight1.1 fluorescence from VTA brain slices during light stimulation. Red-light stimulation increased ntsLight1.1 fluorescence in VTA slices of Chrimson mice, but not in ‘sensor only’ mice (Extended Data Fig. 6b–d). Additional optical control experiments revealed that the increase in ntsLight1.1 fluorescence reaches a maximum at 10 ms red-light pulse widths suggesting ntsLight fluorescence reflects dynamics of NTS release rather than total light delivered to tissue. Delivering blue-light or red-light stimulation in isolation was insufficient to increase ntsLight1.1 fluorescence (Extended Data Fig. 6e,f).
ntsLight2.0 measurements in cell culture assays
Cell culture preparation
HEK 293 T cells (ATCC, CRL-3126; not authenticated; not tested for mycoplasma contamination) were plated and concurrently transfected with pCMV-ntsLight2.0 using Lipofectamine 2000 (Invitrogen, 2980874) according to the manufacture’s protocol. 24 h after transfection, cells were lifted using trypLE Express (Thermo Fisher, 12604021), pelleted (200 g for 2 min) and resuspended in 1 ml culture media containing DMEM (Gibco, 11995-065), fetal bovine serum (Gibco, 26140079) and Pen-Strep (Gibco, 15140148). Cells were then plated onto 4-chamber glass-bottom dishes and imaged the next day.
Spectral measurements
For spectral analysis to determine the optimal excitation wavelength for ntsLight2.0, we used the Leica Stellaris 8 confocal microscope to perform both excitation and emission spectrum measurement. After washing each plate with HBSS (Sigma Aldrich, H8264-500ML), 90 µl of imaging media with 1× HBSS (Fisher, 14175103) and 10 mM HEPES buffer was added to the centre of each quadrant. For emission spectrum measurement, we used λ-scan mode (xyλ) by exciting at 470 nm and imaged with a range of emission wavelength from 480–610 nm with 10 nm step size and 10 frame accumulation. For excitation spectrum measurement, we used excitation lambda scan mode (xyΛ) by exciting with white light laser in a range of wavelength from 440–540 nm with step size at 10 nm. The detection range of the detector precedes the excitation wavelength during the lambda scan emission wavelength. For emission and excitation spectrum with neurotensin, 10 µl of 20 µM NTS was added prior to imaging. Analysis was done using custom code to calculate change in fluorescence (ΔF/F) with before (apo) and after (+NTS) ligand addition. Fluorescence changes were then normalized to the maximum fluorescence in each group (Extended Data Fig. 7b).
Primary hippocampal neuron with antagonist imaging experiment
Glass-bottom 96-well plates (Cellvis) were coated with 0.1 mg ml−1 of poly-d-lysine (Sigma, P6407-5MG) overnight. Plates were washed with UltraPure Distilled Water (Invitrogen, 10977015) and air dried. E18 rat primary hippocampal neurons (BrainBits, https://tissue.transnetyx.com/E18-Rat-Hippocampus_4; not authenticated; not tested for mycoplasma contamination) were dissociated and plated with 38 thousand cells per well in FBS-based neuronal medium containing Neurobasal Plus Medium (Gibco, A35829-01), FBS, GlutaMAX (Gibco, 35050-061) and B27 Plus (Gibco, A3582801). On the next day, medium was removed and replaced with FBS-free neuronal media. On DIV4, half of the neuronal media was changed with new media containing virus AAV9-hSyn-ntsLight2 and removed three days later. Neuronal cultures were imaged on DIV12. Immediately before an imaging experiment, stock peptide solution was prepared in a 96-well treatment plate and serial dilutions (from 300 µM to 3 pM final in HBSS) were prepared across each row. 1 nM and 100 nM final concentration of SR 142948 A (Sigma, SML0015) were then added to the treatment plate. Before adding drug treatment, the 96-well assay plate were washed with HBSS three times and 50 µl imaging medium (vehicle) was added to each well. Baseline imaging was done using ImageXpress Micro Confocal High-Content Imaging System with MetaXpress software (version V6.6.3.55) using a 20× objective and capturing four regions of interest per well. Next, 50 µl of ligand per well from the treatment plate was transferred to the assay plate. After 10 min incubation, the same sites were re-imaged using the same settings. For titration controls without antagonist, only neurotensin from 300 µM to 3 pM dissolved in HBSS were used. Blank controls with vehicle were present in every condition. The images were exported and analysed using a custom MATLAB script (available at https://github.com/lintianlab) to determine changes in fluorescence (ΔF/F). Segmentation was performed on individual images and a mask highlighting the membrane of the HEK293T cells was generated. Pixel intensities were obtained from the mask-highlighted area and the ΔF/F values for each well were calculated and exported (Extended Data Fig. 7c).
Fibre photometry recordings using ntsLight2.0
Signal recording and processing
ntsLight2.0 transients were measured using a custom-built fibre photometry (FIP) system63. In brief, fluorescent signals were obtained by stimulating cells expressing ntsLight2.0 with a 470 nm LED (60 μW at fibre tip). 470 nm LED light signals were released at 20 Hz and light emission was recorded using a sCMOS camera that acquired video frames containing the fibre (5 mm in length, NA 0.48, 400 μm core, Doric Lenses). A TTL signal generated by an Arduino Uno board was used to synchronize the camera and the FIP signal. Video frames were analysed online, and fluorescent signals were acquired using a custom acquisition code (available at https://github.com/handejong/Fipster) and later analysed in GraphPad Prism (version 10.3.1).
Opto-photometry experiments
ntsLight2.0 signals in the VTA were recorded during optogenetic stimulation of NAcLat cells. Specifically, mice were placed in a head-fixed apparatus and connected to fibreoptic patch cords. A 640 nm collimated diode laser was controlled by an Arduino Uno, and laser stimulation times were recorded in the FIP acquisition system with a TTL signal. Each session had 30 trials, 60-s duration, composed of a short laser pulse followed by a delay period. For analysis, we generated peri-event plots of the z-scored data around the laser stimulation onset and analysed the timeframe of −10 to +30 s around the laser stimulation onset across all trials. We then quantified AUC at specific time points: baseline (−3 to −1 s), during laser stimulation (0 to 2 s) and post-laser stimulation (3 to 5 s).
In vivo validation
NAcLat laser stimulation produced bidirectional ntsLight2.0 transients in the VTA composed of a fast negative peak that occurred during laser stimulation (0 to 2 s) followed by a slower positive peak (3 to 5 s). We performed several experiments to test which component of the signal reflects a change in NTS release: First, we compared the observed response in mice expressing both Chrimson and ntsLight2.0 (sensor) to mice that lack one of these components (that is, sensor only, Chrimson only). The positive peak observed in the post-laser stimulation period was increased only when both sensor and Chrimson were expressed (Extended Data Fig. 7d–h). Second, we tested the sensitivity of the signal to varying stimulation patterns. When testing different stimulation durations (1, 3 or 5 s, 10 mW intensity, 20 Hz) we observed that the post-laser stimulation signal had the strongest increase during the 3-s stimulation duration and a moderate change with 1- or 5-s durations (Extended Data Fig. 7i–l). Increasing the stimulation intensities (0.5, 5 or 10 mW intensity for 3 s, 20 Hz) produced a proportional increase in sensor signal (Extended Data Fig. 7m–p). Third, we analysed the sensor signal in response to laser stimulation (3 s, 10 mW, 20 Hz) following intraperitoneal injection of an NTSR1 antagonist (SR48692, Sigma Aldrich, 5 mg kg−1) or an equal amount of DMSO, which were injected 10 min before recording onset. We found that the slower positive, but not the fast negative peak, was blocked by the NTSR1 antagonist (Extended Data Fig. 7d–u), suggesting that laser stimulation produces a brief artefact that is followed by an increase of NTS release. While the nature of the artefact is currently unclear, it is also observed in recordings of other neuropeptide sensors, such as recently developed opioid sensors72, and adenosine sensor (GRABAdo)73. Future experiments are required to test if recruitment of additional neurochemical signalling processes during stimulation may suppress NTS release. Lastly, to test the sensitivity of the sensor to NTS, we continuously recorded the sensor for 20 min, and then the mice were injected with an NTSR1 agonist (PD149163, Sigma Aldrich, 0.3 mg kg−1, intraperitoneal injection). A TTL signal was delivered to the FIP acquisition system from a button pressed on an Arduino Uno board and the signal was recorded for an additional 50 min. The signal was aligned to injection timepoint, detrended and Z-scored averaged using a custom MATLAB code. AUC was analysed during the baseline period (−500 to 0 s before injection) and post injection (1500 to 2000 s). We observed a significant increase in sensor signal in response PD149163 but not saline injection (Extended Data Fig. 7v–x).
Single-cell patch RNA sequencing
Sample collection
The procedure was described previously74. To minimize interference with subsequent molecular experiments, only a small amount of intracellular solution (∼1 μl; not autoclaved or treated with RNase inhibitor) was used in the glass pipette during electrophysiological recordings. Before and during recording, all surface areas—including manipulators, microscope knobs and computer keyboard—that the experimenter needed to contact during the experiment were cleaned with RNaseZAP solution (Sigma). After whole-cell patch-clamp recordings, the cell’s cytosol was aspirated via the glass pipette used during the recording. Although the aspirated cytosol may have contained genomic DNA, our choice of cDNA preparation, which involved poly-A based mRNA selection, virtually eliminated the possibility of genomic contamination in the RNA-sequencing data. Cell collection microtubes were stored on ice until they were used. For sample collection, we quickly removed the pipette holder from the amplifier headstage and used positive pressure to expel samples into microtubes containing 1 µl cell collection buffer (1× Lysis Buffer and RNase inhibitor from Takara’s SMART-seq kits) while gently breaking the glass pipette tip. The sample was spun briefly, snap-frozen on dry ice and stored at −80 °C until further processing.
cDNA library preparation
As described previously74. Single-cell mRNA was processed using Takara’s SMART-Seq v4 and SMART-Seq Single Cell kit according to the manufacturer’s protocol. In brief, the samples were reverse transcribed to cDNA and subsequently amplified. The samples were purified with AMPure XP beads (Beckman Coulter), and the quality and quantity were analysed on a Fragment Analyzer (Advanced Analytical). Library preparation was performed using Nextera XT DNA Sample Preparation Kit (Illumina) as described in the protocol. In short, cDNA samples were fragmented and amplified using index adapters. The samples were then purified using AMPure XP beads and quality and quantity was assessed with the Fragment Analyzer (Advanced Analytical). Following library preparation, cells were pooled and sequenced using Illumina Novaseq 6000 with 150 bp paired-end reads.
Bioinformatics
After sequencing, raw reads were de-multiplexed using Illumina bc12fastq (version 2.20), and pseudo-aligned to the Ensembl GRCm38.95 reference transcriptome and normalized using kallisto (version 0.45.1). All data analysis was performed using Python (version 3.6.7) and R (version 3.5.1). For quality control, we calculated the median absolute deviation of each cell for the reads and the gene counts. Cells with less reads or gene counts than three times the median absolute deviation from the median were excluded. Differential gene expression analysis was performed using edgeR (version 3.24.3). Only genes that were expressed in at least 5 cells with a CPM value above 15 and the average CPM value has to be higher than 4 to be considered for differential gene expression analysis.
Identification of gene families
The Gene Ontology (GO) terms for identification of specific gene families was obtained from QuickGO platform (https://www.ebi.ac.uk/QuickGO/).
Synaptic molecules gene family: GO:0005179 hormone activity, GO:0032098 regulation of appetite, GO:0007218 neuropeptide signalling pathway, GO:0007269 neurotransmitter secretion and GO:0007268 chemical synaptic transmission.
Feeding gene family: GO:0007631 regulation of feeding behaviour and GO:0007631 feeding behaviour.
Synaptic gene family: GO:0043083 synaptic cleft, GO:0097060 synaptic membrane and GO:0099536 synaptic signalling.
Ion channels gene family: GO:0006816 calcium ion transport, GO:0006817 phosphate ion transport, GO:0006814 sodium ion transport, GO:0006811 monoatomic ion transport and GO:0006813 potassium ion transport.
Endoplasmic reticulum gene family: GO:0005783 endoplasmic reticulum.
Vesicle fusion family: GO:0031338 regulation of vesicle fusion, GO:0006906 vesicle fusion and GO:0098992 neuronal dense core vesicle.
Transcription gene family: GO:0010468 regulation of gene expression, GO:0031564 transcription, GO:0090293 antitermination nitrogen catabolite regulation of transcription, GO:0045990 carbon catabolite regulation of transcription, GO:0000409 regulation of transcription by galactose and GO:0046015 regulation of transcription by glucose.
Histology and microscopy
Immunohistochemistry
Immunohistochemistry was performed as described previously26,64. In brief, after intracardial perfusion with 4% PFA in PBS, pH 7.4, the brains were post-fixed overnight and coronal brain sections (50 or 100 μm) were prepared. Sections were stained overnight in a primary antibody solution: rabbit anti-TH (Millipore), chicken anti-GFP (Abcam), rabbit anti-DS Red (Living Colors), all at 1:1,000 dilution. Twenty-four hours later, sections were stained for 2 h in secondary antibody solution (goat anti-rabbit Alexa Fluor 546, (all Thermo Fisher Scientific), goat anti-chicken Alexa Fluor 488 (Abcam), all 1:750). Stained sections were mounted with DAPI-containing mounting medium on microscope slides. Image acquisition was performed with Zeiss LSM710 laser scanning confocal microscope using 20× or 40× objectives or on a Zeiss AxioImager M2 upright widefield fluorescence/differential interference contrast microscope with charge-coupled device camera using 5×, 10× and 20× objectives. Zen Software 2.3 (Zeiss) was used for acquiring confocal and epifluorescence images of brain slices. Images were analysed using ImageJ (NIH, 64-bit Java 1.8.0_172). Sections were labelled relative to bregma using landmarks and neuroanatomical nomenclature as described in The Mouse Brain in Stereotaxic Coordinates65. All images presented with multiple colours represent a composite of images collected with different excitation wavelengths.
Fluorescent in situ hybridization
Fluorescent in situ hybridization experiments were conducted using a commercially available RNAscope Multiplex Fluorescent Reagent Kit V2 (ACD Bio). Brains were extracted and snap-frozen by submerging them into frozen isopentane (−70 to −50 °C). They were stored in an airtight container in an −80 °C freezer. 16 μm coronal NAcLat brain slices were prepared using a cryostat, placed on Superfrost Plus microscope slides (Fisher Scientific) and stored in a −80 °C freezer. On the next day, brain slices were fixed in 4% PFA in PBS (30 min) followed by an ethanol dehydration procedure (20 min). Slices were then bathed in hydrogen peroxide (10 min), followed by protease IV from the RNAscope kit (15 min). Next, probe mixes were made for Nts (Mm-Nts-C2), Ntsr1 (Mm-Ntsr1-C2) or Th (Mm-Th). A custom-made probe (Mm-Nts-O1-C2), targeting only axon 4 of NM_024435.2 (Nts) was designed to assess NTS expression after conditional knockout of Nts in Ntsflox mice (Fig. 4a–e). Probe mixes were applied to the brain slices for hybridization (2 h at 40 °C). After amplification of the signal (using AMP1, AMP2 and AMP3 from the RNAscope kit), channel C1 was developed using green Opal 520 (Akoya Biosciences, USA) and channel C2 was developed using orange Opal 570 (Akoya Biosciences). Finally, nuclei were stained using DAPI (from the RNAscope kit) and brain slices were sealed with ProLong Gold Antifade mountant (Thermo Fisher Scientific) and a glass coverslip. Images were taken using a confocal microscope (LSM710, Carl Zeiss) at 5 different z depths (spanning 4.4 μm), and images were flattened by taking the maximum projection across the z direction. ROIs were identified using a machine learning-based segmentation algorithm nucleAIzer based on the DAPI channel75. The amount of visible mRNA across the DAPI-identified region was used as a proxy for total mRNA in the cell. All identified regions of interest were manually sorted by an investigator who was blind to virus expression, diet, and probe mix. ROIs were removed if they: (1) showed overlap with other regions of interest; or (2) were segmented inadequately by the algorithm. The remaining cells were analysed based on the percentage of DAPI-positive pixels that were also positive for targeted mRNA or based on average fluorescence of targeted mRNA in DAPI-positive cells. To adjust for potential differences in staining and/or image quality, we compared pixels in all regions of interest to background fluorescence levels in each image. To do this, we first established a ‘null distribution’ that quantifies the distribution in pixel intensity values for cells putatively negative for targeted mRNA. Each cell’s distribution of pixel intensities was compared to the null distribution for the targeted mRNA and a correlation coefficient R was calculated. If the R of a cell’s distribution compared to the null distribution was less than 0.85, then a cell was labelled as positive for the targeted mRNA. For experiments in Extended Data Fig. 5i–m, mice exposed to regular diet (REG), 4-week HFD, or mice switched from a 4-week HFD to a 3-week regular diet were injected with a retrograde tracer (fluorescent retrobeads, red) into the VTA. 10 days later, the brains were extracted, and Nts mRNA was assessed in DAPI-positive NAcLat cells labelled with retrobeads.
Statistics and reproducibility
Main effect
Student’s t-tests (paired and unpaired), one-way or two-way ANOVA tests, and mixed-effect model analyses (for normally distributed data) and Friedman, Kruskal–Wallis or Mann–Whitney test (for non-normally distributed data) were used to determine statistical differences using GraphPad Prism 9 (version 9.5.1) and 10 (version 10.3.1) (Graphpad Software). Differential gene expression analysis was performed using Python (version 3.6.7), R (version 3.5.1) and edgeR (version 3.24.3). Wilcoxon’s signed-rank test (α set to 5%) and MATLAB (version R2024a) were used for the analysis of in vivo electrophysiology data.
Multiple comparisons
When a main effect or interaction were reported, Holm–Šídák (for normally distributed data) or Dunn (for non-normally distributed data) post hoc analysis were applied. Spearman correlation coefficient and linear regression were used to measure the strength and direction of the linear relationship between two variables. Statistical significance was denoted by *P < 0.05, **P < 0.01, ***P < 0.001. Data are presented as mean ± s.e.m. for parametric tests, and as median, 25th percentile and 75th percentile for non-parametric tests.
Replication
Several experiments were replicated in at least two technical replicates from each of at least two mice and similar results were obtained. For example, establishing the HFD mouse model (Fig. 1a–d), optogenetics (Figs. 2 and 4i) and NTS-OE (Fig. 5f–l and Extended Data Fig. 10e–k). For anatomical experiments, wherever representative examples are shown (Fig. 4g,k and Extended Data Figs. 1a–d, 3a,b,n,q–t, 4a,b, 6h, 7aa,ab, 8e,f,i,j and 9a,d), similar results were obtained in at least two technical replicates from each of at least two mice.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.