Ethics and inclusion statement
The research process was a collaboration between Europe-based and US-based researchers; citations reflect the global nature and interest of coronavirus infections. Roles and responsibilities were defined between the authors based on their specific area of expertise to ensure the highest quality standards. Animal studies were approved by local ethics committees (see the designated sections). No human participants were involved. All experiments using pathogens were conducted in the appropriate biosafety-containment-level laboratories.
Statistics and reproducibility
In Fig. 3e, representative images are shown of H&E-stained left lung lobes of Syrian golden hamsters infected with SARS-CoV-2 and vehicle or JNJ-9676 treated. In this experiment, a full cross-section of the left lung of each of the five animals per group was assessed by a skilled pathologist.
In Extended Data Fig. 2g, uncut western blots are shown of purified M proteins. These blots were generated once as a quality control of the protein obtained.
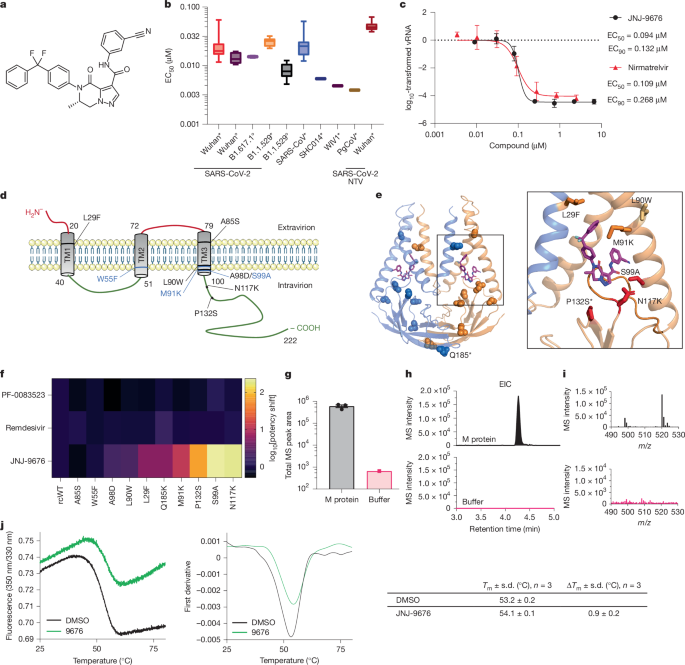
In Extended Data Fig. 6a, a micrograph from the SARS-CoV-2 M–FabB–JNJ-9676 data collection is shown. To obtain this representative image, 12,988 images were taken.
Compounds
The synthesis of JNJ-9676 is described in patent WO-2024/008909 and in the Supplementary Methods. Molnupiravir was ordered at MedChemExpress (HY-135853) and nirmatrelvir was synthesized according to literature procedures59. For in vitro experiments, JNJ-9676, molnupiravir or nirmatrelvir was dissolved in 100% dimethyl sulfoxide (DMSO) as a 5–100 mM stock. For in vivo experiments, JNJ-9676 was dissolved in 100% polyethylene glycol 400 (PEG400) as stocks of 75, 25 or 8.33 mg ml−1, molnupiravir was dissolved in 100% PEG as a stock of 300 mg ml−1 and nirmatrelvir as a stock of 250 mg ml−1.
Cells
VeroE6-eGFP cells were cultures as described previously32. Human epithelial cell line A549 stably expressing hACE2 (A549-hACE2) were obtained from InvivoGen for SARS-CoV-2 and SARS-CoV experiments, or from the American Type Culture Collection (ATCC, CCL-185) for experiments with zoonotic viruses. The cells were cultured as instructed. Pooled donor nasal epithelial cells grown in air–liquid interface format were obtained from Epithelix as a fully differentiated culture and maintained in MucilAIR medium (Epithelix). All cells were maintained at 37 °C in 5% CO2 unless otherwise noted. All cell cultures were checked for mycoplasma contamination and found negative.
Viruses
SARS-CoV-2 strains B1 (BetaCov/Belgium/GHB-03021/2020, EPI_ISL_407976), Delta B.1.617.2 (hCoV-19/Belgium/rega-7214/2021, EPI_ISL_2425097) and Omicron B1.1.529 BA.1 (hCoV-19/Belgium/1-SPL21-p1/2021, EPI_ISL_7413964) were obtained from the University of Leuven, Belgium.
SARS-CoV (Frankfurt strain FFM1; GenBank Accession Number: AY291315) was obtained from Goethe University.
Virus stocks were obtained after six passages in VeroE6-eGFP cells, after which stocks were aliquoted, flash-frozen, and stored at −80 °C.
Recombinant viruses, derived from infectious clones of PgCoV GD/2019, RsSHC014, and WIV-1 expressing nanoluciferase, were derived and isolated as working stocks as previously described60,61,62.
Antiviral assays
SARS-CoV-2 (B1 strain) and SARS-CoV
JNJ-9676 antiviral activity and compound toxicity against SARS-CoV-2 (B1 strain) and SARS-CoV (FFM1 strain) was determined in a high-content imaging (HCI)-based infection assay in A549-hACE2 cells as described previously63.
SARS-CoV-2 (B1 strain, Delta variant and Omicron BA.1 variant)
Antiviral activity of JNJ-9676 against the SARS-CoV-2 B1 strain, Delta variant and Omicron BA.1 variant in VeroE6-eGFP cells was described previously64.
Cytotoxicity was evaluated on day 5 in treated but uninfected cells using an MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) reduction assay65.
Zoonotic sarbecoviruses
The antiviral assays against Pg-CoV, WIV-1 and SHC014 (zoonotic sarbecoviruses) were conducted in A549-hACE2 cells, as previously described61,62,66,67. Antiviral assays against other human coronaviruses are detailed in the Supplementary Methods.
SARS-CoV-2 (B1) in air–liquid interface MucilAIR cultures
JNJ-9676 or vehicle (0.2% DMSO) was prepared in MucilAIR medium (Epithelix) and added on day 1 to the basal compartment of 24-well MucilAIR plates (Corning Costar clear PS plates, Merck) with pooled nasal epithelial cells. Then, 1 h later, the inserts were infected with SARS-CoV-2 (multiplicity of infection (MOI) of 0.1, B1 strain) for 1 h at 37 °C followed by three PBS washes. At 24 and 48 h, apical washes were collected. After 48 h.p.i., cells were lysed using 200 µl of RLT buffer (Qiagen). Automated RNA extraction was performed using the MagNA Pure instrument (Roche) using the MagNA Pure 96 DNA and Viral NA Small Volume Kit for the apical washes and the MagNA Pure 96 Cellular RNA Large Volume Kit for cell extracts. For the apical washes, an external lysis step (Roche lysis buffer) was included before the RNA extraction. One-step reverse transcription quantitative polymerase chain reaction (RT–qPCR) was performed on extracts using the LightCycler Multiplex RNA Virus Master kit (Roche) and SARS-CoV-2 primers and probe (located in nucleocapsid gene; https://stacks.cdc.gov/view/cdc/84525) and in-house designed β-actin primers and probes. Absolute quantification was performed using a logarithmic dilution series of SARS-CoV-2 nucleocapsid RNA fragment standard (in-house generated), on the LightCycler 480 real-time PCR instrument (Roche).
Toxicity was assessed by exposing non-infected inserts to the same concentration of JNJ-9676 as for antiviral treatment by TEER measurements using an EVOM3 (World Precision Instruments), representative of the cell layer’s integrity or health. Brefeldin (0.3 µM; internally synthesized) was used as a toxicity control.
The data were further analysed using GraphPad Prism v.8.
Viral yield reduction assay using SARS-CoV-2 Omicron BA.1 variant
A549-hACE2 cells (8,000 cells per well, 96-well black polystyrene tissue-culture-treated plates (Sigma-Aldrich)) were seeded onto pre-spotted DMSO-dissolved compound in a nine-point dilution series. Columns containing DMSO were used as controls. On day 2, the cells were infected for 2 h with SARS-CoV-2 virus (MOI, 0.1) after which the cells were washed with PBS, the compound was refreshed, and the plates were incubated for an additional 48 h at 37 °C. On day 4, a cytotoxicity read-out was performed, using the ATPlite reagent and a Viewlux instrument (PerkinElmer). In parallel, the supernatant was collected from the inoculated plates for RNA extraction using either the MagNA Pure instrument (Roche) and the MagNA Pure 96 DNA and Viral Small Volume Kit, or QIAamp Viral RNA Mini kit (Qiagen). One-step RT–qPCR was performed using the LightCycler Multiplex RNA Virus Master kit (Roche), and SARS-CoV-2 primers and probe as described above.
Antiviral activity of JNJ-9676 against site-directed mutant viruses
IVRS experiments with JNJ-9676 and the generation of site-directed mutants of SARS-CoV-2 are described in the Supplementary Methods and Supplementary Table 1. The impact of these mutations on the antiviral activity of JNJ-9676 was assessed by an HCI-based antiviral assay63. An overview of the MOI used is presented in Extended Data Table 5. The analysis was performed with Phaedra HCI analysis software (v.1.0.10.202309011029). The fold change in EC50 for a mutant virus compared with the recombinant WT virus was determined. Calculated potency shifts were transformed to logarithmic scale and visualized as a heat map using GraphPad Prism v.9.5.1.
Cloning, protein expression and purification of SARS-CoV-2 M
The gene encoding SARS-CoV-2 M protein (1–222, UniProt: P0DTC5) was synthesized and cloned into a pcDNA3.4 vector, with an added C-terminal linker sequence, an ALFA-tag and a C-tag (SNSLEVLFQGP-SRGGSGAAAGSGSGSGSPSRLEEELRRRLTE-GS-EPEA).
SARS-CoV-2 M was transfected into Expi293F cells (Invitrogen) according to the manufacturer’s protocol and incubated at 37 °C with 8% CO2 for 72 h. The cells were collected by centrifugation at 1,000g, washed with 1× PBS, flash-frozen and stored at −80 °C.
Cell pellets of SARS-CoV-2 M were thawed and resuspended in lysis buffer (20 mM HEPES pH 7.5, 250 mM NaCl, 5% glycerol (v/v), protease inhibitor (Roche), 50 U ml−1 of nuclease). The cell suspension was homogenized using a glass Dounce homogenizer and then lysed using a M110Y microfluidizer (Microfluidics). The cell lysate was centrifuged at 167,900g for 1 h to collect the membranes. The membranes were resuspended in the same buffer and solubilized by adding lauryl maltose neopentyl glycol (LMNG, Anatrace) and cholesteryl hemisuccinate (CHS, Anatrace) to a final concentration of 1% and 0.1% (w/v), respectively. After incubation for 2 h at 4 °C, the supernatant was collected by centrifugation at 167,900g for 30 min and incubated with C-tag resin (Thermo Fisher Scientific) for 2 h at 4 °C with gentle rotation. The resin was washed with 10 column volumes (CV) of wash buffer (20 mM HEPES pH 7.5, 250 mM NaCl, 1.25% glycerol (v/v), 1 mM EDTA, 0.0025% LMNG (w/v), 0.00025% CHS (w/v)). The protein was eluted using 3 CV of elution buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 1.25% glycerol (v/v), 1 mM EDTA, 0.0025% LMNG (w/v), 0.00025% CHS (w/v), 3 mM C-tag peptide (Vivitide)). The protein was further purified by size-exclusion chromatography on the Superose 6 Increase 10/300 GL column (Cytvia) in buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.001% LMNG (w/v), 0.0001% CHS (w/v), 0.00033% glycol diosgenin (GDN; w/v)).
Cloning, protein expression and purification of FabB
The sequence encoding the heavy chain of FabB2 was modified to contain a truncated C terminus to block Fab dimer formation (-CKPCICTVPEVSS) and cloned into the pcDNA3.4 vector with an added C-terminal 6×His-tag containing a linker (GS-GS-HHHHHH). The sequence encoding the light chain of FabB2 was cloned into a pcDNA3.4 vector with an added N-terminal gLUC signal sequence (MGVKVLFALICIAVAEA).
pcDNA3.4 vectors containing FabB heavy chain and light chain were co-transfected into Expi293F cells (Invitrogen) according to the manufacturer’s protocol and incubated for 96 h at 37 °C with 8% CO2.
Conditioned medium was loaded onto a 10 ml HisTrap excel column (Cytvia) at a flow rate of 8 ml min−1. The column was washed with 6 CV of wash buffer (20 mM sodium phosphate pH 6.5, 150 mM NaCl, 20 mM imidazole) and eluted with over 5 CV using a 39.2–500 mM imidazole gradient prepared in buffer (20 mM sodium phosphate pH 6.5, 150 mM NaCl, 500 mM imidazole). Peak fractions of FabB were subsequently purified on to a HiLoad 16/600 Superdex 75 pg column (Cytvia) in buffer (20 mM sodium phosphate pH 6.5, 150 mM NaCl).
Purification and formation of SARS-CoV-2 M–FabB complex
SARS-CoV-2 M and FabB were mixed at a 1:2.5 ratio and incubated on ice for 1 h. The SARS-CoV-2 M–FabB complex was loaded into a Superose 6 Increase 10/300 GL column (Cytvia) with buffer (20 mM HEPES pH 7.5, 150 mM NaCl, 0.001% LMNG (w/v), 0.0001% CHS (w/v), 0.00033% GDN (w/v)). The peak fractions containing the SARS-CoV-2 M–FabB complex were pooled, 100 µM JNJ-9676 was added and incubated for 1 h on ice. The sample was diluted to 0.2–0.8 mg ml−1 with size-exclusion chromatography (SEC) buffer containing 100 µM JNJ-9676 for cryo-EM.
Nano differential scanning fluorometry
Experiments were performed in a total volume of 10 µl. A Prometheus NT.Plex instrument (NanoTemper Technologies) was used to measure the melting temperatures. The samples were prepared in a 384-well plate with 0.5 mg ml−1 purified recombinant SARS-CoV-2 M and 100 µM of JNJ-9676 in 20 mM HEPES pH 7.5, 150 mM NaCl, 0.001% LMNG (w/v), 0.0001% CHS (w/v), 0.00033% GDN (w/v) and 1% DMSO (v/v). The samples loaded into standard‐grade glass capillaries were measured under a temperature range of 25–95 °C with a temperature gradient of 1 °C min−1, and the intrinsic protein fluorescence at 330 and 350 nm was recorded. The data were analysed using PR.ThermControl v.2.1.6 (NanoTemper Technologies) (technical replicates ≥ 3).
Offline ASMS
The offline ASMS experiment consisted of the preparation of three sample types: compound QC, protein target (M protein) and no-protein control (breakthrough).
For the preparation of SEC filter plates for offline ASMS, 130 µl of pre swollen Bio-Gel P10 resin slurry was added to each well of a low-protein-binding Millipore HTS 384 HV filter plate (hereafter, size-exclusion plate) with a 0.45 µm Durapore (PVDF) membrane (MZHCN0W10). The size-exclusion plate was placed into a 4 °C refrigerated centrifuge, centrifuged at 1,000g for 2 min and the flowthrough was discarded. Each cartridge was then washed a total of four times using 50 µl buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl, 0.001% LMNG, 0.0001% CHS, 0.00033% GDN and 2% DMSO, whereby the flowthrough from each wash was discarded after centrifugation at 1,000g for 2 min. The ASMS assay plate was prepared using an Echo acoustic liquid handler, and an aliquot of 20 nl of 5 mM compound dissolved in 100% DMSO was transferred from the source plate into four separate wells of a 384-well, natural, polypropylene V-bottom plate (781280). An aliquot of purified recombinant M protein stock solution was thawed on ice, then diluted using assay buffer to a working concentration of 5 µM and 2% DMSO. Then, 20 µl of the resulting working protein stock was dispensed into three wells containing compound to yield a final concentration of 5 µM (3 technical replicates). To control for compound breakthrough of the SEC resin, either in-solution or through micelle partitioning, a separate working stock was prepared without protein and dispensed as a 20 µl aliquot into the remaining compound well. The plate was centrifuged at 1,000g for 1 min at room temperature and incubated at 25 °C for 30 min.
All of the samples were transferred to the size-exclusion plate, which was quickly centrifuged at 1,000g for 2 min at 4 °C to minimize compound breakthrough. The resulting flowthrough was diluted with 15 µl MS-grade water (Honeywell) to reduce the detergent concentration and centrifuged further at 2,000g for 5 min at room temperature to collect any insoluble precipitate.
The compound QC sample was prepared separately without additional handling, whereby a 5 nl aliquot of 5 mM compound in DMSO was transferred from the source plate into a 384-wellplate and combined with 25 µl of 49% acetonitrile, 2% DMSO solution.
All liquid chromatography–mass spectrometry (LC–MS) analyses were performed on an Agilent 1290 Infinity II uHPLC system coupled to an Agilent 6545XT qTOF using the Agilent MassHunter (v.10.0) software. A 4 µl sample injection was loaded with water as a loading solvent onto the reversed-phase column (2.1 × 35 mm ACQUITY UPLC BEH C18 column, 130 Å, 1.7 µm), heated to 40 °C. LC separation was performed using mobile phases consisting of water (solvent A) and acetonitrile (solvent B), each containing 0.2% formic acid. The LC method used a constant flow rate of 0.1 ml min−1 and consisted of a 1 min wash with 5% solvent B, a steep gradient from 5% to 20% B over 0.1 min, a subsequent shallow gradient from 5% to 95% B over 1.9 min, followed by a hold for 1 min and a return to 5% B in 0.1 min with a 0.9 min hold. The MS instrument was operated in positive polarity mode with centroided data acquisition, where the source was set to a 350 °C drying gas temperature and 13 l min−1 drying gas flow rate; 375 °C sheath gas temperature and 12 l min−1 sheath gas flow rate; capillary voltage of 3,300 V; nozzle voltage of 500 V; nebulizer pressure of 50 psi; fragmentor of 125 V; and skimmer of 50 V. A reference mass solution consisting of purine and HP-0921 (Agilent, G1969-85001) was prepared according to the manufacturer’s instructions and infused to apply automatic mass correction to all spectra acquired from 110 to 1,100 m/z at a rate of 1 spectrum per second.
MS data processing was performed using Agilent MassHunter Qualitative Analysis (v.10.0), where the [M+H]+, [M+Na]+, and [M+K]+ masses were extracted and merged using a mass error tolerance window of 3 ppm.
Cryo-EM
QuantiFoil Au 1.2/1.3 300 mesh grids were subjected to glow discharge using the PELCO easiGlow Discharge Cleaning System. A total of 3 µl recombinant M protein sample (0.8 mg ml−1), prepared as described above, was applied to the EM grids, which were vitrified with a Vitrobot (Thermo Fisher Mark IV) using the following settings: blot time 4 s, blot force 0, wait time 0 s, inner chamber temperature 4 °C, and 100% relative humidity. Flash-freezing in liquid ethane cooled by liquid nitrogen was performed. Cryo-EM data collection was automated on the 200 kV Thermo Scientific Glacios microscope controlled by EPU software. Micrographs were taken at ×105,000 magnification using a Facon4 detector (Gatan) in counting mode. Each 6 s exposure recorded 40 frames with a total dose of 40 e− Å−2. The calibrated physical pixel size for all digital micrographs was 0.910 Å. All details corresponding to individual datasets are summarized in Extended Data Table 6.
Cryo-EM data collection and image quality were monitored using cryoSPARC Live v.3.2. Image preprocessing steps, including patch motion correction, patch contrast transfer function (CTF) estimation, blob particle picking (100–200 Å diameter) and extraction, were performed simultaneously. A total of 12,988 raw micrographs was recorded during a 4-day data collection session using the Glacios microscope. Acceptable 2D classes served as templates for particle repicking. One round of live 2D image classification yielded approximately 1.2 million good particle images. These particles were used for 3D reconstruction. The first round of five starting 3D models were calculated, resulting in one major 3D class, followed by a second round of four 3D classes. One major class underwent non-uniform 3D refinement and local refinement using 484,610 particles and was further refined to a 3D EM map with an average resolution of 3.06 Å.
Resolutions were estimated by applying a soft mask around the protein complex density using the gold-standard (two halves of data refined independently) FSC = 0.143 criterion. Before visualization, all density maps were sharpened by applying different negative temperature factors along with the half maps and used for model building. Local resolution was determined using ResMap. Detailed statistics about the cryo-EM data processing can be found in Extended Data Fig. 6a–f.
Cryo-EM model building, refinement and validation
Human SARS-CoV-2 M protein dimer (short form) in a complex with FabB (PDB: 7VGS) was used as the initial model for atomic model building of the EM map. For the M–FabB complex model building, the M protein was manually built using COOT68. The FabB was fitted into the 3D map using Chimera and then further refined manually with COOT followed by real-space refinement in Phenix69. Detailed data collection and structural refinement statistics are provided in Extended Data Table 6 and Extended Data Fig. 6g. Structure representations were generated using Pymol (v.2.0)70 and Chimera71.
Pre-exposure Syrian golden hamster model
Housing conditions and experimental procedures were performed according to project 062/2020, approved by the ethics committee of KU Leuven, Belgium license number LA1210186. The hamster infection model of SARS-CoV-2 has been described previously72. Statistical power analysis as well as the limitations of the study size warranted 5 animals per group to obtain statistical significance in Syrian golden hamster studies. After arrival, the animals were randomly assigned to groups. No blinding was performed during the experiment. Female hamsters (Janvier Laboratories), 8–10 weeks old, were inoculated intranasally with 50 μl containing 2 × 106 TCID50 SARS-CoV-2 B1 (day 0). Animals were treated according to the schedule (Fig. 3a), with vehicle or JNJ-9676 (75, 25 or 8.33 mg per kg per dose, formulated in 100% PEG400). Animals were dosed BID at 08:00 and 16:00. Viral RNA and infectious virus levels in the right lung were quantified using RT–qPCR and end-point virus titration, whereas left-lung samples were subjected to histopathological scoring, as described previously72 (Fig. 3b–e).
For histological examination, the fixed lung tissue sections (5 μm) were analysed after staining with haematoxylin and eosin and scored blindly for lung damage by an expert pathologist. The scored parameters, (cumulative score, 1 to 3), were as follows: congestion, intra-alveolar haemorrhagic, apoptotic bodies in the bronchus wall, necrotizing bronchiolitis, perivascular oedema, bronchopneumonia, perivascular inflammation, peribronchial inflammation and vasculitis.
All statistical analyses were performed in GraphPad Prism v.9.5.0 and validated using R (v.3.6.1). A log10 transformation was applied to the lung viral-load data (RNA and infectious virus) to approximate normality. The mean differences between the treatment groups and the vehicle group were estimated using the one-way analysis of variance with Šídák’s multiplicity correction to account for multiple testing.
In the case that normality could not be assumed for the outcome variable or in case of lung histopathology, the nonparametric Kruskal–Wallis test by ranks was applied. The post hoc Dunn’s test with the Benjamini–Hochberg’s multiplicity correction was applied to account for multiple testing. A significance level of 0.05 was used.
Post-exposure Syrian golden hamster model
Housing conditions and experimental procedures were performed as described and approved by the ethics committee of Johnson & Johnson Research & Development (Belgium), license number LA1100119. Statistical power analysis as well as the limitations of the study size warranted 5 animals per group to obtain statistical significance in Syrian golden hamster studies. After arrival, the animals were randomly assigned to groups. No blinding was performed during the experiment. Female Syrian golden hamsters (Janvier Laboratories) aged 8–10 weeks were anaesthetized by isoflurane inhalation and inoculated intranasally with 100 μl of PBS containing 1 × 104 TCID50 SARS-CoV-2 (day 0). The animals were treated orally starting at 10, 24 or 48 h.p.i. and continued to be dosed BID at 10 h intervals with vehicle or JNJ-9676 (75 mg per kg per dose in PEG400) (Fig. 3f). The animals were dosed BID at 08:00 and 16:00. On day 4 after infection, the hamsters were euthanized by CO2 inhalation. Whole right lungs were homogenized by bead disruption using the Precellys homogenizer (Bertin Instruments). Viral RNA and infectious virus levels were quantified in the lung homogenate supernatant by RT–qPCR and end-point virus titration, respectively (Fig. 3g,h). RNA was extracted using the MagNA Pure 96 DNA and Viral NA Small Volume Kit following the Viral NA universal SV 4.0 protocol (Roche). RT–qPCR was performed using the LightCycler Multiplex RNA Virus Master kit (Roche), and SARS-CoV-2 primers and probe as described above. For end-point titrations, a 1:10 serial dilution of the lung homogenate was prepared in 1× MEM (without phenol red (Thermo Fisher Scientific) supplemented with 2% FCS (Biowest), 2 mM alanyl-glutamine (Sigma-Aldrich) and 0.04% gentamicin (Thermo Fisher Scientific). This dilution series was then added to confluent Vero E6 cells in a 96-well plate and incubated for 72 h at 37 °C. The infectious viral titres of the samples were determined by microscopically scoring the virus-induced cytopathic effects and quantified as the TCID50 ml−1 according to the Reed–Muench calculation method73. The TCID50 ml−1 values were normalized to the total weight of the right lung and expressed as TCID50 per mg tissue.
The statistical analysis was performed as described above.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.