The average rate at which germinal centre (GC) B cells accumulate mutations in their immunoglobulin (Ig) loci is estimated to be one per 1,000 bases per cell generation14,15,16, corresponding to about a two-thirds chance of acquiring at least one mutation per daughter cell when considering the roughly 700-bp length of the combined Ig heavy-chain (Ighv) and light-chain (Igkv or Iglv) variable regions. Because deleterious mutations—resulting in stop codons or reductions in antibody affinity or structural integrity—in general greatly outnumber affinity-enhancing mutations6,17, a crucial challenge for the GC is to identify multiple beneficial mutations while avoiding most, if not all, deleterious ones. Early mathematical models of GC mutation and selection proposed that a solution to this problem would be for cells to alternate phases of stochastic somatic hypermutation (SHM) and antigen-driven selection, the latter serving to purge the population of deleterious mutations while enriching for beneficial ones before allowing cells to mutate again. Assigning these two stages to the GC dark zone (DZ) and light zone (LZ), respectively, led to the establishment of the ‘cyclic re-entry’ paradigm, now the standard framework for how GC selection operates11,12,13.
More recently, we and others showed that the degree to which B cell clones expand varies considerably between individual GCs7 and with the amount of T cell help provided8,10. The fastest-growing clones undergo marked selective sweeps—which we termed clonal bursts—in which the descendants of a single B cell take over an entire 2,000-cell GC structure in a matter of days7. A large body of mechanistic work indicates that such bursts derive from B cells that received exceptionally strong proliferative signals from T follicular helper (TFH) cells present in the LZ. Help from TFH cells triggers B cells to re-enter the DZ and undergo multiple rounds of proliferation by ‘inertia’ — that is, in the absence of further T cell-derived signals and thus in the absence of affinity-based selection8,9,10,18,19,20,21,22. Because such bursts require multiple rounds of inertial cell division not interspersed with affinity-based selection, the expectation, given a uniform rate of mutation per cell cycle, is that bursting B cells would rapidly accumulate deleterious mutations that would lead to precipitous declines in antibody affinity and integrity11,12,13. Thus, if a constant rate of SHM is assumed, the existence of clonal bursts would be incompatible with cyclic re-entry.
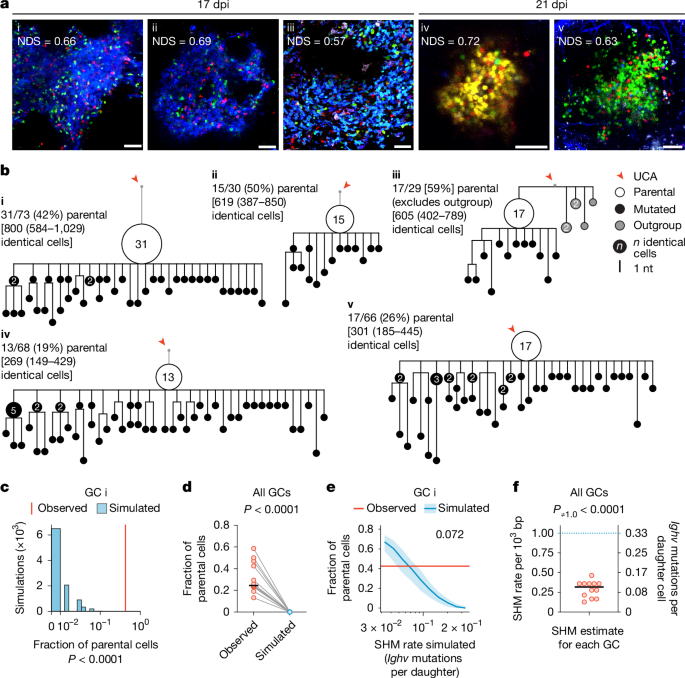
To resolve this apparent conflict, we began by examining the mutational patterns of clonal bursts at single-GC resolution. To this end, we used AicdaCreERT2/+.Rosa26Confetti/Confetti (AID-Brainbow) mice7,23,24, in which multicolour fate-mapping of a Brainbow25 allele in B cells expressing activation-induced cytidine deaminase (AID) allows for easy identification of GCs containing large clonal bursts by microscopy. We immunized these mice with the model antigen chicken IgY in alum adjuvant to form GCs, triggered Brainbow recombination by treatment with tamoxifen starting on day 5 post-immunization (dpi) and scanned popliteal lymph nodes (pLNs) collected at 17 or 21 dpi for single-coloured GCs with a normalized dominance score (NDS; a measure of the proportion of cells of the dominant colour within the GC) greater than 0.5, indicative of clonal-burst-like expansion7,26. Using single-GC microdissection7, we isolated B cells from 12 such GCs and sequenced their Ighv genes. We then used sequences belonging to the dominant clone in the GC to build mutational phylogenies in an unsupervised manner using the gctree algorithm27. In gctree phylogenies, B cells that fail to mutate while rapidly proliferating appear as large nodes containing multiple cells with an identical Ighv sequence, followed by a variable number of descending branches. The ratio of the abundance of B cells at the parental node (defined as the node with the greatest number of identical sequences in the bursting phylogeny, which probably represents the B cell that initiated the clonal burst) over the abundance of its mutated descendants is inversely related to the mutational probability per division (Extended Data Fig. 1a). Dominant phylogenies derived from the 12 sequenced clonal bursts (9 from 17 dpi and 3 from 21 dpi) consisted uniformly of structures with large parental nodes, with an average fraction of parental cells of 0.32 ± 0.14 (s.d.) (Fig. 1a,b and Extended Data Fig. 1b,c). By contrast, larger phylogenies derived from five control GCs with NDS < 0.3 (including one phylogeny (GC vi) that was obtained from the same lymph node as GC i and in which a sister lineage of the same B cell clone was expanded) were generally more branched and, although quantitative comparisons are not possible given that a parental node by definition cannot be assigned to such lineages, generally lacked nodes with a large number of identical cells (Extended Data Fig. 1d,e).
a, Multiphoton images of single-coloured GCs indicative of clonal bursting, obtained at 17 or 21 dpi (10–14 days after the final dose of tamoxifen). The normalized dominance score (NDS) represents the fraction of cells belonging to the dominant colour, adjusted for fluorescent cell density7 (see Supplementary Data 1). Scale bars, 50 µm. b, Phylogenetic trees of Ighv sequences from the GCs in a. For each GC, the parental cell fraction and estimated counts of identical cells (±95% confidence interval (CI)), extrapolated to a 2,000-cell GC (in square brackets), are provided. Outgroup cells in GC iii are excluded from calculations. See additional trees in Extended Data Fig. 1b,c. nt, nucleotide; UCA, unmutated common ancestor. c, Observed parental fraction for GC i (red line) compared with distributions from 10,000 simulated clonal branching processes, matched to extrapolated GC sizes (blue bars). P values reflect the number of simulations with parental fractions at or above the observed value. d, Observed fractions of parental cells from 12 GCs (b,c and Extended Data Fig. 1b,c) compared with simulated fractions assuming a constant SHM rate of 0.333 Ighv mutations per daughter. P value by paired t-test. Horizontal black line shows the median. e, Median (±interquartile range) parental fractions from parameter sweep simulations of GC i with SHM rates increasing geometrically from 0.033 to 0.333 Ighv mutations per daughter (see Extended Data Fig. 2). The red line indicates the observed parental fraction for GC i. The number denotes the simulated SHM rate at which the median parental fraction most closely matched the observed fraction in vivo. f, Estimated SHM rates for 12 clonal bursts. P value by one-sample t-test against the theoretical SHM rate of 0.333 Ighv mutations per daughter. Horizontal black line shows the median.
Linear extrapolation of the measured fraction of parental cells to a 2,000-cell GC7 indicated that the clonal expansions in our 12 single-coloured GCs must contain on the order of several hundred B cells with identical Ighv sequences (Fig. 1b and Extended Data Fig. 1c). This is, at face value, incompatible with the premise of a constant mutation rate of 1 per 103 bases per daughter cell14,15,16: assuming a uniform mutation rate and an approximately two-thirds chance of a descendant retaining the parental sequence (here we are looking at the Ighv segment only, which is close to 300 bp in length), the anticipated outcome after a 10-division burst is that, on average, only 18 (that is, 2/310 × 210) of 1,024 (210) B cells would retain the parental sequence. To formally test this intuition, we used a birth–death process simulation28 to generate phylogenies with a constant Ighv mutation probability of one mutation per 1,000 bp incorporated (or one-third for each daughter after a cell division). For each GC in Fig. 1a, we ran simulations until the number of cells matched the empirical estimates from the sequenced clones (square brackets in Fig. 1b and Extended Data Fig. 1c). We performed 10,000 such simulations for each GC, randomly sampled cells from each simulation to match the number of cells found in the original in vivo phylogeny and then calculated the fraction of parental-type cells in each simulated sample (Fig. 1c,d and Extended Data Fig. 2a). In only 21 of the 120,000 simulation-derived samples did the parental fraction reach that observed in the original GCs in vivo. All 21 such instances were simulations of GC xi (Extended Data Fig. 1c), which had an exceptionally low fraction of parental cells and thus is likely to represent a burst that took place earlier in the course of the GC response. The median parental fraction for all simulations was zero, compared with 0.25 for experimentally measured GCs (Fig. 1d). A parameter sweep showed that the SHM rate that most closely approximated the observed parental fraction for each GC was, on average, 0.10 (range 0.043–0.16) mutations per Ighv region per daughter cell (equivalent to 0.30 (0.13–0.46) mutations per 103 bases per generation; Fig. 1e,f and Extended Data Fig. 2b,c). Thus, clonal-burst phylogenies exhibit SHM rates that are between one-half and one-eighth of previously established average GC mutation rates14,15,16.
Our findings raise the hypothesis that GC B cells actively downregulate SHM during clonal bursting. To test this, we sought to measure SHM in the presence or absence of perturbations that promote the inertial proliferation of GC B cells that drives bursting21 (Extended Data Fig. 3a). We first examined a genetic model in which GC B cells carry a Burkitt-lymphoma-associated gain-of-function mutation in the Ccnd3 gene (Ccnd3T283A). This mutation slows the nuclear export and proteasomal degradation of the cell-cycle regulator cyclin D3, leading to moderate increases in DZ inertial cell cycling and in the size of the DZ compartment itself21,29,30. Previously, we used double-nucleotide pulsing10 to obtain the average percentages of wild-type (WT) and Ccnd3T283A/+ GC B cells entering S phase within the DZ in a 30-minute window (3.63% ± 0.26% (s.d.) and 5.80% ± 0.23%, respectively)21. On the basis of these data, we estimated that T283A-mutant GC B cells had undergone 67% more inertial cycles in the DZ by 10 dpi (13.5 versus 8.1) (Extended Data Fig. 3b). Assuming a uniform mutation rate per division cycle among DZ B cells, we would expect Ccnd3T283A/+ B cells to accumulate more mutations than WT cells (5.01 ± 0.84 versus 3.01 ± 0.67 mutations per cell, respectively) (Extended Data Fig. 3c). However, the observed SHM distributions in WT and Ccnd3T283A/+ GC B cells were indistinguishable (Extended Data Fig. 3c–e), and, accordingly, the estimated mutation rate of Ccnd3T283A/+ GC B cells was significantly lower than that of WT GC B cells (Extended Data Fig. 3f). More precisely, whereas mutations among WT cells occurred at a rate close to the predicted one14,15,16 (0.38 ± 0.09 mutations per daughter, or 1.01 ± 0.15 mutations per 103 bases), mutations in Ccnd3T283A/+ GC B cells were substantially less frequent than expected (0.22 ± 0.05 mutations per daughter, or 0.59 ± 0.08 mutations per 103 bases) (Extended Data Fig. 3f). Therefore, despite increasing the number of DZ cells engaging in inertial cycling, Ccnd3T283A/+ mice did not show a concomitant increase in mutation accrual over time, pointing to a reduced mutation rate per cell division.
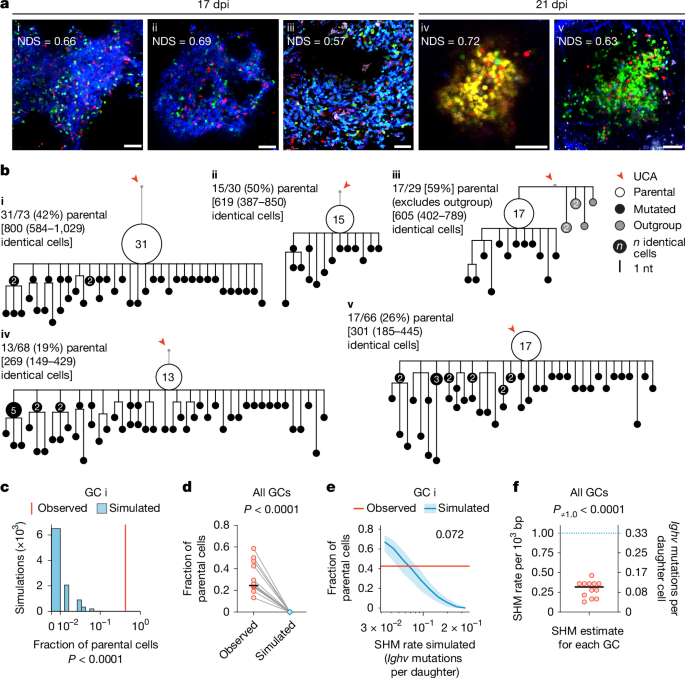
We next used a model in which we could deliberately force several B cell lineages to undergo multiple rounds of inertial cycling in the DZ en masse8 (Fig. 2a). We combined this experimental model with a genetically encoded tracker that measures cell division as a function of the dilution of mCherry fluorescent protein10. WT hosts received an adoptive transfer of B1-8hi B cells (specific for 4-hydroxy-3-nitro-phenylacetyl; NP), of which 5% express mCherry under a doxycycline (DOX)-sensitive promoter, as well as the receptor DEC-205 (encoded by the gene Ly75), whereas the remaining 95% genetically lack DEC-205 expression (Ly75–/–). We then immunized these mice with NP coupled with ovalbumin (OVA) to generate GCs. Treating mice with established GCs with an antibody specific for DEC-205 coupled to OVA (anti-DEC-OVA) leads to enhanced presentation of OVA to TFH cells by DEC-205-expressing GC B cells only, forcing these to interact with TFH cells, migrate to the DZ and then rapidly undergo several rounds of proliferation in the absence of LZ selection8,9,10. We analysed GC B cells 72 h after treatment with anti-DEC-OVA (10 dpi), after cells have undergone substantial proliferative expansion in the DZ (Fig. 2b) but mostly before they have initiated a subsequent round of selection in the LZ.
a, Schematic of the anti-DEC-OVA experiment. b, Ratio of DEC-205+ to DEC-205– B1-8hi GC B cells in anti-DEC-OVA-treated and untreated mice. c, Flow-cytometry plot showing estimated number of cell divisions, as assessed by mCherry dilution (factor of 2 per generation), in B1-8hi GC B cell populations. Estimated mean division numbers are shown above the histogram. DEC-205+ cells used in the analysis were sorted using the mCherrylow gate. d, Mean mutation counts per Ighv + Iglv region per mouse. Observed mutation counts are compared to predictions based on a uniform mutation rate of 1 in 1,000 bases per daughter cell. Each symbol represents one of four mice per group from two separate experiments. Horizontal black lines show the median. e, Mutation distributions in pooled samples from d. Data for individual mice are in Extended Data Fig. 4a. f, Inferred SHM rate for individual mice from d. Horizontal black lines indicate inverse variance-weighted mean with s.d. accounting for variances within individual mice. g, Ratio of DEC-205+ to DEC-205– B1-8hi GC B cells in anti-DEC-OVA-treated and untreated mice as in a. The white circle indicates the median for each group. Each symbol represents one of 6–9 mice per group from 2 independent experiments. h, DZ/LZ distribution of DEC-205+ and DEC-205– B1-8hi GC B cells in anti-DEC-OVA-treated mice. i, Mean change in mutation counts per Ighv + Iglv region in anti-DEC-OVA-treated and untreated mice, relative to the 0-h time-point mean. The white circle indicates the median for each group. j, Distribution of mutations in pooled 0-h, 48-h and 72-h samples from i. Data for individual mice are in Extended Data Fig. 4b. P values by Student’s t-test (f,g,i), Kolmogorov–Smirnov test (e,j), or paired t-test (d,h).
Setting the mCherry fluorescence intensity measured at 7 dpi as the baseline, we calculated the extent of cell division from 7 to 10 dpi, with or without anti-DEC-OVA-induced proliferation. On average, cells underwent 3.9 divisions without the treatment and 5.2 divisions with the treatment (Fig. 2c). We then sorted B1-8hi cells to directly measure SHM loads by droplet-based paired Ig sequencing. Specifically for the day-10 sample treated with anti-DEC-OVA, we sorted DEC-205-expressing B1-8hi cells that had low levels of mCherry expression (corresponding to approximately 7.5 divisions, on average), to avoid confounding effects from lineages that underwent fewer divisions (Fig. 2c). Assuming a mutation rate of one per 1,000 bases for each daughter cell, we would expect to gain, by day 10, 3.5 and 9.3 Ighv + Iglv mutations (around 700 bases) in untreated and treated mice, respectively (Fig. 2d). Whereas the SHM accumulation from 7 to 10 dpi in GC B cells from untreated mice aligned with our predictions, averaging 1.6 mutations per cell, the empirical outcome for mice treated with anti-DEC-OVA was notably distinct. Not only did their GC B cells accumulate fewer mutations than predicted, but these cells also exhibited a slightly lower mutation count than their untreated counterparts (mean 1.1 mutations per cell; Fig. 2d,e and Extended Data Fig. 4a). Accounting for division rates, these mutational loads corresponded to a decrease in the SHM rate from 0.67 ± 0.10 Ighv + Iglv mutations per daughter cell (or 0.95 ± 0.12 per 1,000 bases) in untreated mice to 0.12 ± 0.03 (or 0.17 ± 0.04 per 1,000 bases) after treatment with anti-DEC-OVA (Fig. 2f). Together, our results indicate that SHM rates per daughter cell decrease when GC B cells are forced, either genetically or pharmacologically, to undergo more inertial DZ cycles—once again arguing against a uniform rate of mutation per round of cell division.
To gain insight into the mechanisms that underlie the suppression of SHM in inertially cycling cells, we first quantified the appearance of mutations over time in the anti-DEC-OVA setting. Although DEC-205-sufficient B cells proliferated substantially and accumulated markedly in the DZ between 0 h and 48 h after anti-DEC-OVA treatment (Fig. 2g,h), SHM in this population ceased completely during this expansion period, after which it resumed at a near-normal rate (Fig. 2i,j and Extended Data Fig. 4b). Thus, the suppression of SHM after treatment with anti-DEC-OVA is not due to an even slowdown in mutation rates throughout the response, but rather to a temporary cessation of SHM during inertial cycling, followed by a return to the baseline rate at around the time of LZ re-entry.
A comparison of B cell gene expression at 48 and 72 h after anti-DEC-OVA treatment ruled out downregulation of Aicda (which we confirmed by flow cytometry using an AID-GFP fusion reporter31) as the mechanism for this pause in SHM (Extended Data Fig. 5a,b). Moreover, expression of downstream effectors of SHM (Ung, Neil1, Neil3, Apex1, Apex2, Msh2, Msh6, Polh, Hmces and Fam72a; refs. 32,33,34,35,36,37,38) was generally higher rather than lower at 48 h after anti-DEC-OVA treatment, although fold changes were modest (Extended Data Fig. 5a). There was also no detectable downregulation of Igh mRNA or of Jh4 intronic RNA (a real-time indicator of Ighv transcription), suggesting that transcription of the Ighv region, a requirement for AID targeting39, is intact during inertial cycling (Extended Data Fig. 5c). By contrast, inertial cycling was associated with a sharp decrease in the proportion of B cells assigned transcriptionally to the G0–G1 phase of the cell cycle (Extended Data Fig. 5d). This is in line with our previously published findings in the Ccnd3T283A mutant21 and indicates that B cells spend less time in the early phases of the cell cycle while undergoing inertial divisions. Given the established restriction of SHM to the earlier cell-cycle stages40,41,42, we hypothesized that B cell lineages undergoing clonal bursting in the DZ would be unable to undergo SHM except during the final post-mitotic phase that takes place between the end of inertial cell cycling and return to the LZ. Such a regimen would be equivalent to undergoing one round of SHM per DZ passage, rather than one per cell division (Fig. 3a). To test this hypothesis, we sought to measure the length of time B cells spend in G0–G1 under conditions of normal or clonal-burst-type cycling.
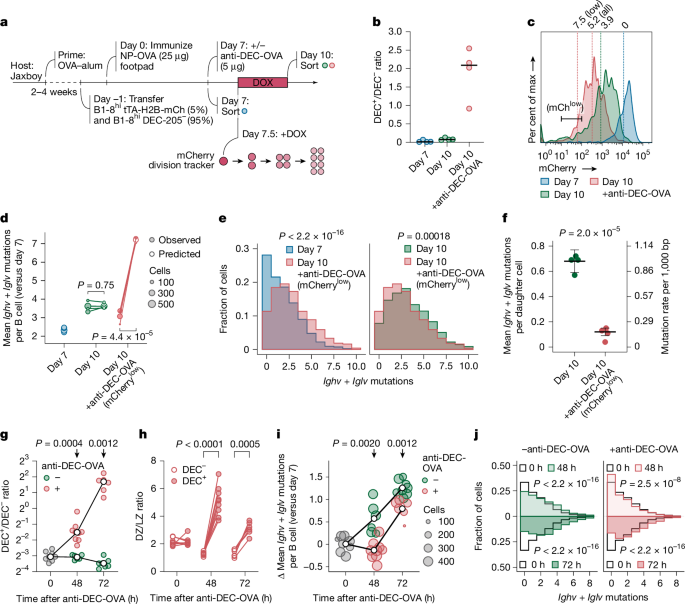
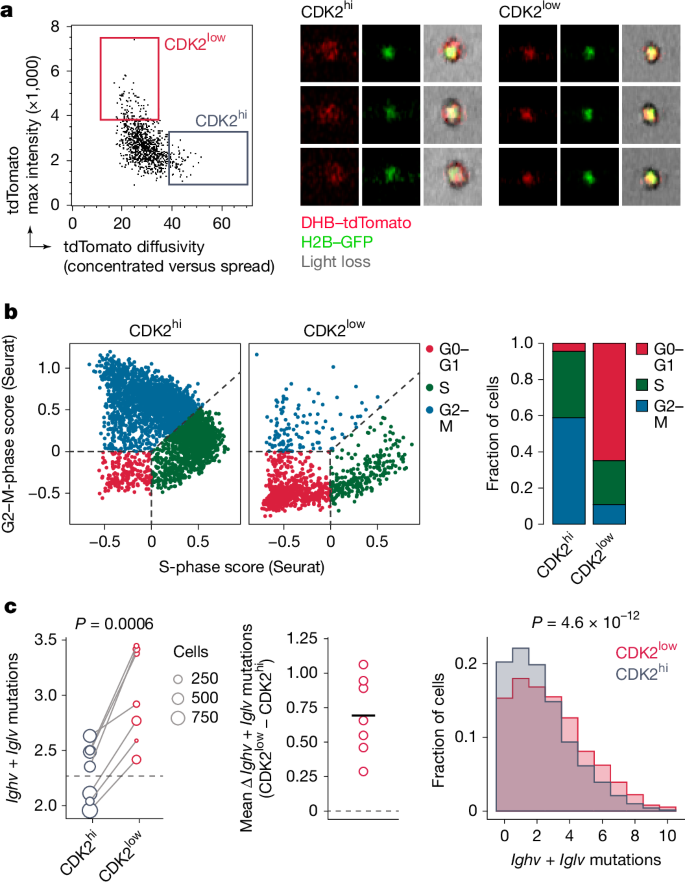
a, Proposed model for AID and/or SHM activity. b, Detecting CDK2 activity through subcellular translocation of a DHB reporter. c, Rosa26DHB-tdTomato knock-in (KI) allele design. Asterisk denotes a silent mutation introduced to prevent sgRNA binding in the KI template. d, DHB–tdTomato (red) and H2B–GFP (green) in cultured GC B cells, with corresponding C/N ratios. Scale bars, 5 μm. e, Snapshots from time-lapse imaging of GC B cells in Nojima cultures expressing H2B–GFP (green) and DHB–tdTomato (red at the top, greyscale at the bottom; Supplementary Video 1). Scale bar, 10 µm. f, CDK2 activity traces (grey) with mean ± s.d. (red). Cells treated with anti-CD40L blocking antibody (blue) provide a CDK2low reference. g, Duration of the C/N ratio below 1.0 (S-phase entry) after anaphase. Each symbol represents one daughter cell. h, DHB–tdTomato (red) and H2B–GFP (green) in GC B cells. CD35 expression (yellow dotted line) delineates the LZ (Supplementary Video 2). Arrowheads in insets highlight nuclear DHB–tdTomato in LZ cells and cytoplasmic expression in DZ cells. Scale bars, 50 µm (top); 20 µm (bottom). i, DHB–tdTomato (grey) after treatment with anti-DEC-OVA. Arrowheads highlight nuclear DHB–tdTomato at 72 h. Scale bars, 20 µm. j, Violin plot of CDK2 activity with box plot overlay (median, interquartile range, range). The grey box indicates the CDK2low state (median of untreated LZ cells). Individual GC data are in Extended Data Fig. 6b,c. k, Fraction of CDK2low cells in paired LZ and DZ cells in untreated GCs and DZ B cells after treatment with anti-DEC-OVA. P values by Student’s t-test. l, Snapshots of DHB–tdTomato (red) and H2B–GFP (green) from intravital time-lapse imaging at 0 h or 36 h after treatment with anti-DEC-OVA (Supplementary Videos 3 and 4). Images are aligned to the time of anaphase, determined by sister chromatid separation (arrowheads). CDK2 activity is indicated for each daughter cell. Scale bars, 10 µm. m, In vivo CDK2 activity traces in DZ cells with or without treatment with anti-DEC-OVA, summarized with mean and s.d. Non-dividing LZ cells (blue) serve as a CDK2low reference. P value by Student’s t-test.
The G0 and G1 phases of the cell cycle are characterized by low activity of cyclin-dependent kinase 2 (CDK2), which, when partnered with cyclin E, promotes progression through G1 and S-phase initiation43. To measure these phases precisely in vivo, we generated mice carrying a real-time reporter of CDK2 activity (Fig. 3b,c). This reporter, based on previously published constructs43,44, consists of amino acids 994–1,087 of human DNA helicase B (DHB) fused N-terminally to the tdTomato red fluorescent protein and inserted into the ubiquitously expressed Rosa26 locus (Rosa26DHB-tdTomato; Fig. 3c). The DHB domain translocates from nucleus to cytoplasm after phosphorylation by CDK2. It therefore localizes to the nucleus when CDK2 activity is absent during G0, gradually exiting to the cytoplasm as cells progress through G1, S and G2 phases43,45 (Fig. 3b,d). This localization-based readout for CDK2 activity, determined as the cytoplasmic-to-nuclear (C/N) ratio of DHB–tdTomato fluorescence (Fig. 3b), allows for monitoring of cell cycle progression with greater time resolution than can be achieved using conventional genetically encoded cell-cycle indicators46 that require transcription, translation and maturation of fluorescent proteins to take place before a signal can be detected. Rosa26DHB-tdTomato mice were crossed to the B1-8hi B cell receptor and a histone 2B (H2B)-EGFP reporter47 to accurately visualize mitotic events.
To test our reporter, we longitudinally imaged DHB–tdTomato+ GC B cells sorted onto feeder cell monolayers expressing CD40L, BAFF and IL-21 (‘Nojima’ cultures), which, in combination, are potent stimulators of GC B cell proliferation ex vivo48,49 (Supplementary Video 1). To follow the behaviour of our reporter during the transition from M phase to the following S phase, we aligned multiple single-cell DHB–tdTomato localization traces in time using anaphase as a reference (Extended Data Fig. 6a). We were unable to observe any cells entering a CDK2low state, in which DHB–tdTomato is fully nuclear, as was the norm for GC B cells cultured in similar conditions but in the presence of a blocking antibody to CD40L (Fig. 3). Thus, when under constant stimulation, GC B cells never fully deactivated CDK2. Instead, immediately after mitosis, all cells exhibited an intermediate level of CDK2 activity, which continued to build and reached a C/N ratio of 1.0 (the reported threshold for the initiation of DNA synthesis43) within a median time of 1.3 h after anaphase, out of an average total cell-cycle duration of 8.7 h (Fig. 3g). Therefore, GC B cells under strong mitogenic stimulation are able to transition rapidly from M to S phases of the cell cycle by maintaining CDK2 activity close to the threshold required for S-phase entry, transiting rapidly through G1 and effectively avoiding a CDK2low G0 stage43.
To verify whether such rapid M-to-S-phase transition was also seen during inertial cycling in vivo, we injected anti-DEC-205-OVA into mice with ongoing GCs seeded with a minor fraction of DHB–tdTomato-expressing B1-8hi B cells, as in Fig. 2a, and imaged GCs both statically and dynamically using intravital imaging windows50,51. Static analysis of untreated GCs revealed that LZ B cells predominantly showed nuclear localization of DHB–tdTomato (median C/N ratio of 0.53; Fig. 3h,j,k, Extended Data Fig. 6b and Supplementary Video 2). Given that LZ B cells remain mainly in a quiescent G0-like state while competing for positive selection signals, we set the median of LZ B cells (0.53) as the threshold to define the CDK2low state. In DZ B cells, by contrast, DHB–tdTomato was frequently enriched in the cytoplasm (median C/N ratio of 1.00 and 18.3% of the cells in the CDK2low state; Fig. 3h,j,k, Extended Data Fig. 6b and Supplementary Video 2), indicative of active proliferation. Treatment with anti-DEC-205-OVA markedly increased the proportion of DZ B cells in active cell cycle (median C/N ratio of 1.2 and 1.1; 4.9% and 1.0% of cells in the CDK2low state at 36 and 60 h after anti-DEC-OVA treatment, respectively), suggesting that inertially cycling DZ B cells mostly avoid a G0-like phase, instead maintaining an intermediate level of CDK2 activity after division (Fig. 3i–k, Extended Data Fig. 6c and Supplementary Video 2). By 72 h after treatment, when B cells are still predominantly in the DZ but anti-DEC-205-OVA-induced inertial proliferation is subsiding21, the median C/N ratio fell to 0.81, with 16% of DZ B cells in a CDK2low state (Fig. 3i–k, Extended Data Fig. 6c and Supplementary Video 2).
Intravital time-lapse imaging of actively cycling DZ B cells showed that, at 36 h after anti-DEC-OVA treatment, C/N ratios in DZ B cells remained steadily above the CDK2low G0 threshold (we were unable to track B cells for long enough to detect the increase in C/N ratios observed in vitro), whereas, in the absence of treatment, C/N ratios continued to decrease after mitosis, reaching the CDK2low threshold within 30 min of anaphase (Fig. 3l,m and Supplementary Videos 3 and 4). Thus, B cells actively undergoing inertial cycling in response to anti-DEC-OVA largely failed to enter a CDK2low G0-like state.
To demonstrate the relationship between SHM and the CDK2low state, we sought to measure the mutational load specifically among CDK2low cells at 72 h after anti-DEC-OVA treatment. We used image-based cell sorting coupled with single-cell mRNA sequencing to isolate GC B cells with nuclear (CDK2low) versus cytoplasmic (CDK2hi) DHB–tdTomato reporter and a DZ transcriptional phenotype (Fig. 4a and Extended Data Fig. 7a–c). We found the expected strong association between nuclear DHB–tdTomato and a G0–G1 transcriptional state (that is, a lack of S- or G2–M-phase gene-expression programs) among DZ B cells (Fig. 4b). Crucially, as predicted by our model, CDK2low DZ cells had on average 0.69 (±0.28, s.d.) additional mutations in Ighv + Iglv, compared with CDK2hi DZ cells from the same sample (Fig. 4c), confirming that somatic mutation indeed takes place when B cells are in a CDK2low state. Together, our data show that SHM is restricted to DZ B cells in the G0-like phase of the cell cycle, which indicates that the suppression of SHM that is evident during inertial cycling is, at least in part, attributable to the widespread skipping of this phase during inertial cell cycling.
a, Sorting gate (left) and examples of GC B cells with nuclear (CDK2low) and cytoplasmic (CDK2hi) DHB–tdTomato reporter as identified by image-based cell sorting (right). See Extended Data Fig. 7a,b for full sort strategy and further examples. b, Left, assignment of cell-cycle phase to CDK2hi and CDK2low DZ B cells by expression of S-phase and G2–M transcriptional signatures. Right, quantification. Cell-cycle categories are assigned using the CellCycleScoring function in Seurat. G0–G1 cells are defined as those lacking expression of S-phase and G2–M-phase transcripts. c, Left, number of Ighv + Iglv mutations in CDK2low and CDK2hi B cells 72 h after treatment with anti-DEC-OVA. Each symbol represents the mean of one mouse and is scaled according to the number of cells sequenced; line is the mean of all CDK2low B cells. P value by paired t-test. Middle, difference between mean CDK2hi and CDK2low B cells in each mouse. Right, distribution of mutations in pooled CDK2low and CDK2hi B cells. Horizontal black line shows the mean. P value by Kolmogorov–Smirnov test.
Finally, to gain insight into how restricting SHM during inertial cycling might affect clonal selection and affinity maturation in GCs, we simulated full GC reactions in silico, whereby we could artificially turn off the ability of GC B cells to suppress SHM during inertial cycling. We adapted an established agent-based model of the GC reaction capable of realistic clonal bursting7,9,26,52 (see Methods and Supplementary Text) to include two scenarios: (i) a strict version of the model in Fig. 3a, in which B cells downregulate SHM to zero during inertial cycling and are completely barred from mutating until after the last round of DZ division before returning to the LZ (LAST); and (ii) a model in which this feature is disabled, and SHM is kept at a constant rate during each round of DZ division (EACH). When cells were allowed to mutate, the expected number of mutations in a descendant cell compared to its parent was set to 2.2, so as to yield an average SHM rate of 0.66 mutations per generation in LAST. We performed a total of 1,000 simulations for each scenario; GCs were simulated for 20 days after initial coalescence (corresponding to approximately 23 days in vivo), and late GCs were removed from analysis when they contracted to fewer than 200 cells.
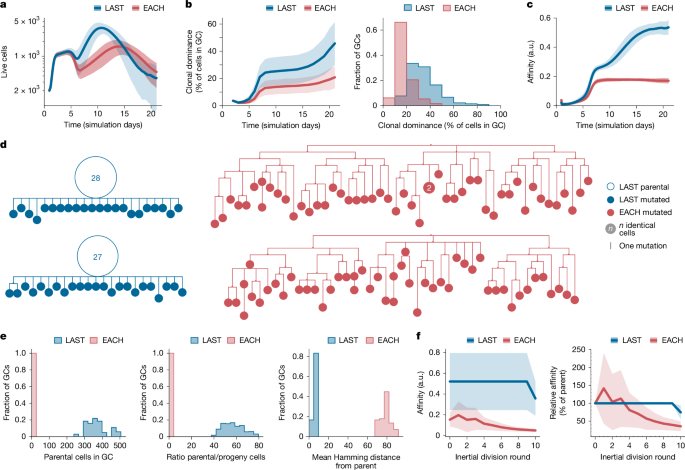
EACH GCs, in which the downmodulation of SHM during clonal bursting is disabled, were smaller in size and kinetically delayed compared with GCs generated by the LAST model (Fig. 5a). Both the rate of clonal expansion—measured as the percentage of each GC accounted for by its largest clone—and the average affinity of GC B cells were markedly higher in LAST GCs (Fig. 5b,c), suggesting that the expansion of high-affinity B cell clones in GCs fails in the presence of constant SHM. To investigate the effects of SHM downregulation at the phylogenetic level, we generated trees for all GCs containing clones with dominance greater than 75% in the LAST and EACH models. To mimic experimental conditions, samples of 100 cells were drawn from each GC, and all cells belonging to the bursting clone were included in the phylogeny. LAST phylogenies exhibited large parental nodes containing multiple cells with identical sequence, whereas EACH phylogenies were more branched and generally lacked expanded nodes (Fig. 5d), qualitatively matching the phenotypes of bursting and non-bursting GCs in our experimental data (Fig. 1b,c and Extended Data Fig. 1). Accordingly, the number of cells in the largest node of each bursting phylogeny was notably higher (193 ± 186 versus 0.69 ± 0.60 cells), and the mutational (Hamming) distance between the bursting cell and its progeny was much lower (0.57 ± 0.12 versus 7.7 ± 0.59 mutations) in LAST compared to EACH (Fig. 5e). To verify the prediction that sustaining SHM rates during inertial cycling would lead to a degradation of affinity in the progeny of a clonal burst, we selected phylogenies that underwent ten consecutive rounds of inertial division in both models, and plotted the average affinity of B cells after each round of division. As expected, clonal bursting in the EACH model led to an average decay in affinity as cells proliferated, whereas bursting lineages in the LAST model maintained higher affinities until the final round of DZ division (Fig. 5f,g). We conclude that removing the constraints on SHM during inertial cycling in silico leads to a marked loss of affinity among the descendants of a clonal burst and an overall failure of affinity maturation.
a–c, One thousand in silico GC reactions were performed for each of two scenarios: EACH, in which B cells can undergo several divisions per DZ round, in a manner that depends on the amount of help they received in the LZ, and are free to mutate after every cell division; and LAST, which works as in EACH except that B cells can mutate only after the last inertial division of a DZ round. a, Number of live GC B cells. b, Percentage of B cells originating from the same founder B cell clone (clonal dominance) over time and frequency distribution of clonal dominance in GCs at day 14. c, Mean affinity of GC B cells in arbitrary units (a.u.) for the 1,000 simulations performed using each model. d, Example phylogenetic trees constructed from a sample of 100 cells drawn from day-14 phylogenies that had undergone ten consecutive inertial cell divisions. e, Quantification of data as in d across all bursting phylogenies. Distribution of bursting phylogenies according to the number of parental cells (left) and ratio of parental to progeny cells (middle) in each phylogeny. Right, mean mutational (Hamming) distance of all cells in the phylogeny to the cell that initiated the burst. f, Left, mean absolute affinity of GC B cells in a.u. Right, change in affinity in relation to the parental node for cells in the course of all bursts reaching ten divisions. Calculations in e,f are for the entire phylogeny rather than for the 100-cell samples shown in d. Shaded areas around the mean indicate s.d.
Somatic mutation and clonal expansion—two of the hallmarks of the GC reaction—are crucial for producing large quantities of high-affinity antibodies, but would pose problems for affinity maturation were they to occur simultaneously11,12,13. We propose that the immune system solves this potential conflict by restricting SHM to a specific stage of the cell cycle that is unavailable to rapidly dividing DZ B cells until the end of inertial proliferation, such that B cells undergo one round of mutation per DZ passage rather than per cell cycle (Fig. 3a). In this framework, clonal bursts closely resemble the behaviour of B cells in the original version of the cyclic re-entry model, undergoing several rounds of mutation-free division before each round of SHM11. However, because this model did not recapitulate the experimentally determined zonal distribution and migration kinetics of average GCs, it was subsequently replaced by its authors by a model in which B cells recycled more frequently between zones, with limited proliferation in each DZ passage12. Our findings suggest that these two versions of cyclic re-entry represent extremes of the same distribution of GC selection outcomes. At one end of the spectrum, weakly selected B cells undergo only a single round of division, followed by SHM, before returning to the LZ. This scenario converges with a model in which SHM takes place after every mitosis, and is roughly consistent with average GC kinetics12, given that, in steady state, GC B cells undergo an average of two divisions per DZ passage10. At the other extreme, clonal bursts represent exceptional deviations from average GC behaviour in which strongly selected B cell lineages expand several hundred-fold in a single DZ passage11. It is in this extreme scenario that the silencing of hypermutation during most division cycles becomes experimentally evident.
Mechanistically, the simplest explanation for an association between inertial cycling and dampened SHM would be that the total time a cell spends in the G0–G1 phase is shortened during inertial expansion, allowing less time for AID to act on Ig genes before the onset of DNA replication. Such a model is in agreement with the relatively slow rate of AID deamination of cytosines in vitro53. However, our findings suggest that the key difference between steady-state and inertially cycling GC B cells is that the latter specifically fail to access a state of low or no CDK2 activity. Emerging views on the regulation of cell-cycle entry indicate that, in continuously cycling cells, low CDK2 activity characterizes a temporary quiescent phase of the cell cycle—G0 or at least ‘G0-like’—which can be skipped entirely in cells that are proliferating very rapidly43. This raises the possibility that specific features of the DZ G0-like state either enhance AID activity or favour error-prone over faithful repair of the uracils it creates in DNA32,33,34,35. For example, it was recently shown that D-type cyclins, which are highly expressed during DZ inertial cycles21, inhibit the recruitment to DNA lesions of components of the error-prone mismatch repair (MMR) pathway54,55, instead favouring more faithful base excision repair (BER) in G0–G1 (ref. 56). Thus, a potential mechanism for the downregulation of SHM during inertial cycles is that high cyclin D activity keeps error-prone MMR at bay. This possibility is in line with our finding that mice in which cyclin D3 is stabilized show decreased SHM rates per cell division. However, it would require an additional mechanism operating during inertial cycling to ensure that AID-catalysed uracils are repaired faithfully by BER, which in GC B cells can also be mutagenic35.
A dynamic rate of SHM in GC B cells had been invoked in previous mathematical models precisely because, in silico, reducing mutational probabilities in high-affinity cells, which are those that divide the most, was found to improve affinity maturation9,57. The mechanism proposed here—namely, that mutation is limited to the last division of each DZ round—aligns with that prediction by reducing the number of mutations per DZ passage specifically in high-affinity cells, which are those most likely to undergo inertial proliferation in the DZ. Nonetheless, completely eliminating SHM during inertial cycles yields phylogenies that are more strongly dominated by the parental node than are those we observed in vivo (compare Fig. 1b and Extended Data Fig. 1c to Fig. 5d). Barring technical explanations related to imprecisions in the timing of clonal bursts in relation to sampling in vivo, this suggests that mutation is strongly suppressed but may not be entirely absent during inertial cycling.
In conclusion, our data support a model in which B cells silence SHM during inertial cycling, so as to undergo one (or possibly a few) SHM cycles per DZ passage, as opposed to one round per cell cycle. This model reconciles two salient features of GC biology—cyclic re-entry and clonal bursting—and explains how GCs can simultaneously achieve the efficient clonal expansion and sequence diversification that are required for antibodies to affinity mature.