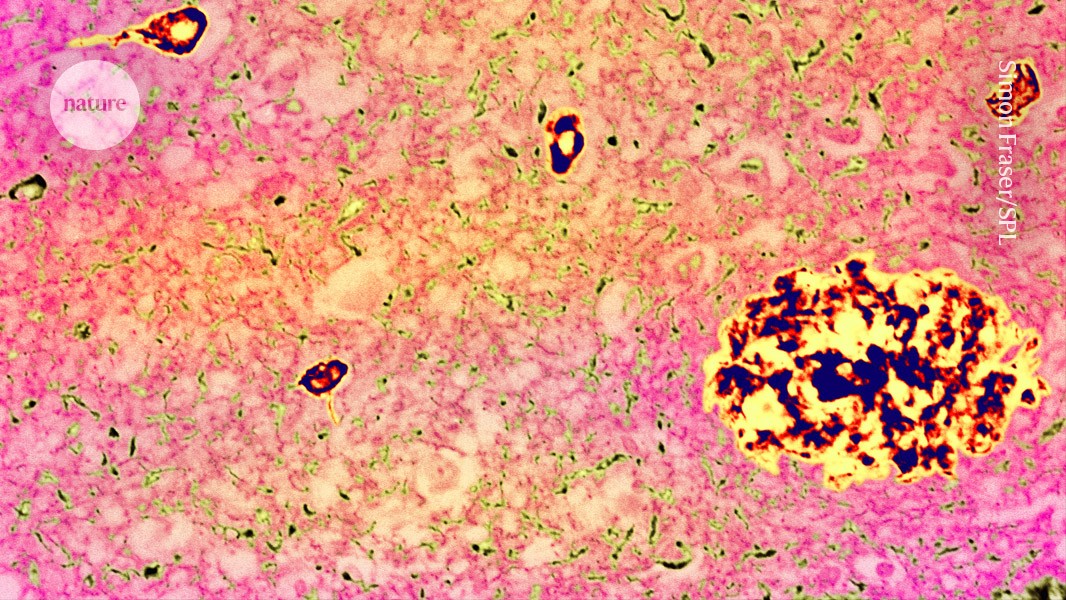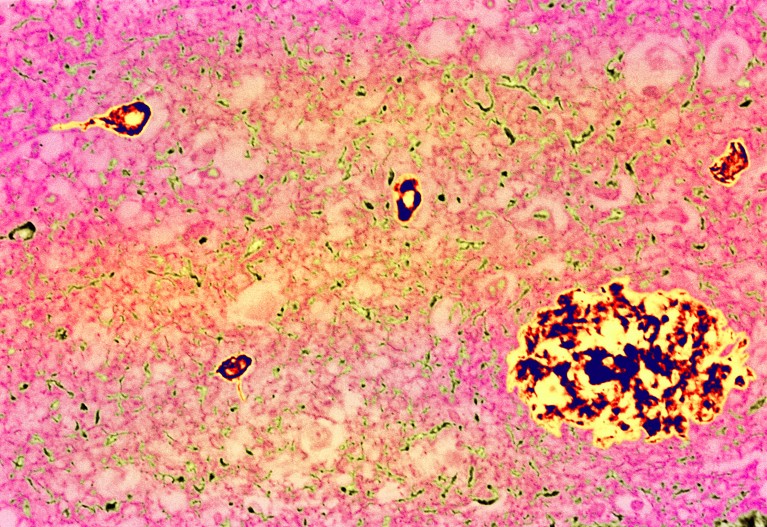
Plaques (yellow-black) in the brains of people with Alzheimer’s disease contain amyloid-β, created by the breakdown of a protein called APP.Credit: Simon Fraser/Science Photo Library
A protein involved in Alzheimer’s disease progression has been linked to normal brain ageing, raising the prospect that researchers could target it to stave off age-related mental decline.
The amyloid-β precursor protein (APP) helps to form links between the brain’s neurons. It has been highly studied because its breakdown creates amyloid-β peptides, which are often present in plaques in the brains of people with Alzheimer’s disease.
What accelerates brain ageing? This AI ‘brain clock’ points to answers
Now, Dario Valenzano, an evolutionary biologist at the Leibniz Institute on Aging in Jena, Germany, and his colleagues have found that knocking out the appa gene, which makes APP, in turquoise killifish (Nothobranchius furzeri) reduces signs of ageing.
“We found that there is probably an overlooked role of amyloid precursor protein in normal brain ageing,” says Valenzano. The work has not yet been peer reviewed and is posted on the bioRxiv preprint server1.
Rapid decline
Turquoise killifish tend to live no more than about 9 months and exhibit rapid age-related brain decline. Valenzano’s team looked inside neurons of aged fish — those about 6 months old — and discovered an accumulation of APP derivatives, including a damaging type of amyloid-β, that aren’t present in 6-week-old fish.
This is different from what happens in Alzheimer’s disease, in which amyloid plaques form outside cells.
Hacking the immune system could slow ageing — here’s how
The researchers then looked at brain samples from people over 80 with and without Alzheimer’s disease. They found a similar accumulation of APP and amyloid-β inside cells in the cortex and the hippocampus in both healthy and diseased brains.
Next, the team used CRISPR gene editing to breed killifish that don’t have the appa gene. Brain tissue studies showed that this slowed cell death and decreased brain inflammation in ageing fish. It also improved the age-related decline in neuronal activity and capacity for learning. “We see quite remarkable rescue of learning in elderly fish,” says Valenzano. “They learn in a way that’s more like younger fish.”
The team demonstrated this by training killifish to associate a burst of air bubbles with a light: when the light comes on, the fish must move away to avoid a bubble burst. The gene-edited fish learnt this faster than normal fish of the same age.