In October, after almost a decade of work, Google licensed its artificial intelligence (AI) model for detecting the eye disease diabetic retinopathy to three health-care technology companies — two in India and one in Thailand. It came with a condition: the firms have to provide six million free AI screenings to people in low- and middle-income countries (LMICs) over the next ten years.
“They will be setting up their own business models, but on the side, they will also be delivering screenings to people who need it the most but can’t afford it,” says Sunny Virmani, project manager at Google Health in Mountain View, California. “Blindness from diabetic retinopathy is completely preventable, and the fact that we have not been able to do effective screening in some of these places shouldn’t be forgiven.”
Nature Outlook: Vision
Of the 43 million people in the world who are blind or visually impaired, almost 90% live in LMICs. There are many social and economic issues that contribute to this: living in rural communities can hinder people’s ability to access health care, and a lack of health literacy can lead some people to think that blindness is a normal and untreatable consequence of ageing. Low-income countries also have only 3–4 ophthalmologists per one million people; in high-income countries, this number is about 76 per one million people.
Using AI to screen for and diagnose eye conditions, such as glaucoma, age-related macular degeneration and diabetic retinopathy could help to lower the burden of visual impairment. Research suggests that the technology reduces the need for specialists, makes care more accessible and improves adherence to follow-up visits. But the real-world cost of AI screening remains uncertain, and hiccups encountered during the deployment of these tools could reduce their effectiveness.
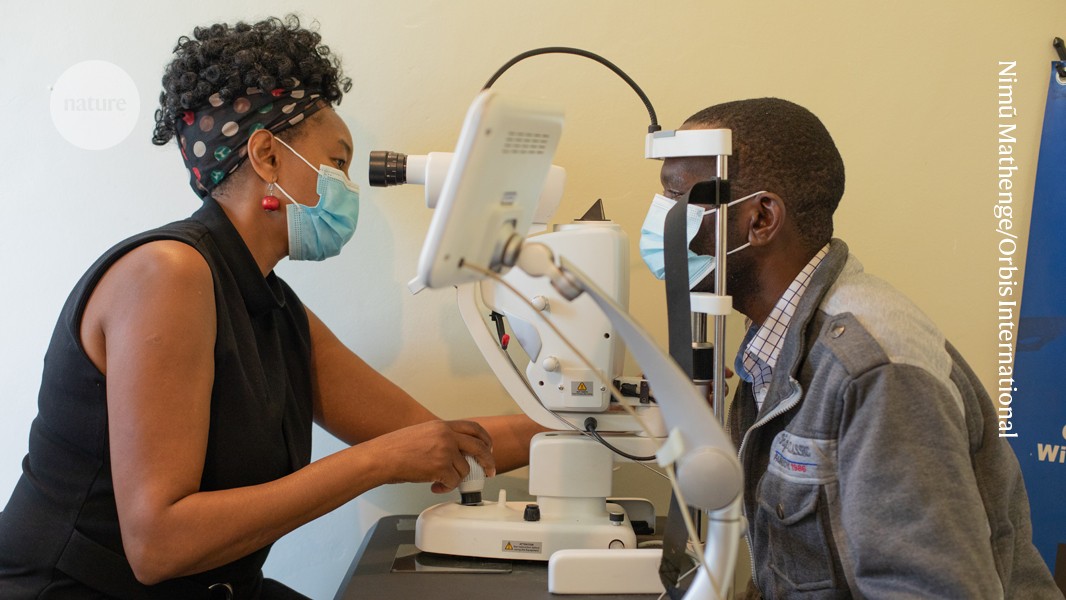
Ophthalmology is primed for the use of diagnostic AI technology. “There is lots of imaging data that has been collected for many years as a routine practice, and that data is perfect for training AI models,” says Charles Cleland, an ophthalmologist and researcher at the London School of Hygiene & Tropical Medicine.
A growing need
For some eye conditions, AI diagnosis is unlikely to move the needle. Cataracts, for example, account for the majority of vision loss, globally, and most people with cataract-related visual impairment live in LMICs. But the bottleneck in tackling cataracts is not diagnosis, but limited access to surgery. A 2016 analysis1 found that surgery rates are as high as 10,000 per one million people in the United States and Europe, but can be fewer than 500 per one million people in sub-Saharan Africa.
AI-assisted screening for diabetic retinopathy, however, could be of considerable benefit. Diabetes is becoming more common in LMICs — by 2045, it is estimated that more than 21 million adults in sub-Saharan Africa will be affected, up from fewer than 9 million in 2019. Diabetic retinopathy develops when excessive sugar in the blood damages blood vessels, which causes fluid to leak into the eye. This can cause changes in vision such as sudden colour blindness or night blindness, floating spots and blurring. Eventually, a person could become blind — but early diagnosis and treatment reduces this risk by about 98%.
Surgery is required only at the most advanced stages of diabetic retinopathy. Before that, the condition can be treated with injections of corticosteroids or anti-vascular endothelial growth factor (anti-VEGF) drugs, or with laser treatments that can reduce retinal swelling and stop blood loss in the eye. These are not straightforward to deliver in LMICs — anti-VEGF drugs must be administered repeatedly in the clinic through injections into the eye, for example. However, if the disease is caught early enough, then simple treatments to manage a person’s blood glucose levels can slow progression — or even prevent a person with diabetes from developing eye problems in the first place.
“A delay in diagnosis of diabetic retinopathy can mean irreversible blindness,” says Cleland. But right now, “only a small number of those people with diabetes get screened”.
Proven technology
In 2014, Dale Webster, director of research at Google Health, and his colleagues began to test AI’s capacity to diagnose disease from medical images. Researchers had just demonstrated that AI image-recognition systems could classify breeds of cats and dogs in images better than humans could. One of Webster’s colleagues was a family friend of ophthalmologist R. Kim, director of Aravind Eye Hospital in Madurai, India. When Kim mentioned that one of the hospital’s main challenges was a lack of specialists to quickly assess a person’s eyes for signs of diabetic retinopathy, the researchers realised that AI could help.
The team spent a couple of years developing a system, known as automated retinal disease assessment (ARDA), that could diagnose the disease as effectively as ophthalmologists in a laboratory setting. To diagnose diabetic retinopathy, physicians and algorithms alike analyse the interior of the back of the eye, known as the fundus. ARDA ingests one image from each eye taken by a specialist camera, which Webster says costs about US$10,000. Use of these cameras is straightforward, and does not require an ophthalmologist.

A portable camera that images the interior of the back of the eye is used alongside AI software to screen for diabetic retinopathy.Credit: Hugh Bassett
The technology seems to be at least as good as ophthalmologists at diagnosing retinopathy. Between 2018 and 2020, the Google team, in collaboration with Paisan Ruamviboonsuk, an ophthalmologist at Rajavithi Hospital in Bangkok, and his colleagues, screened 7,651 people in three regions in Thailand2. Around 30% were subsequently referred to specialists for either diabetic retinopathy or diabetes-related macular oedema — or, based on a separate test, poor vision. For diabetic retinopathy that threatened vision — either early-stage but severe disease, or later-stage disease with blood vessels growing on the retina — ARDA achieved an accuracy of 94.7%.
This study was “a major milestone in providing evidence that these tools are safe and effective”, Webster says. “The next important step is to enable doctors to use it in their current workflows to screen more people so that fewer cases are missed, and fewer people go blind.”
Although Thailand has a national screening programme for diabetes, only about 50% of people with diabetes are assessed for eye disease. This is, in part, because of a lack of trained staff. AI screening tools could help to boost efficiency. A 2023 study of an eye clinic in Bangladesh found that physicians could see about 920 people in about 7 weeks. When clinic staff used an autonomous AI diagnosis system called LumineticsCore, developed by Digital Diagnostics in Coralville, Iowa, 1,189 people were screened over a similar period3.
Another challenge with diabetic retinopathy in LMICs is getting people to a specialist after they are diagnosed. In some low-resource settings, it can take weeks to get images evaluated and graded on a scale of disease severity, and for the results to be returned to an individual. A small study led by Cleland in Tanzania in 2016 found that, after this length of time, only about 25% of people attended their recommended follow-up appointments4.
“In low-resourced settings, patients are there to receive care. It’s hard to ask them to come back again when they have so many other competing issues,” says Jennifer Patnaik, an epidemiologist at the University of Colorado School of Medicine in Aurora. She and her colleagues demonstrated that using AI screening to deliver immediate results to people with diabetic retinopathy in Rwanda could increase the number of people accessing follow-up care5. Around half of those who received results from the Cybersight AI system took up their recommended referral to a specialist straight away — 30% more than when people were made to wait just 3–5 days for a person to grade the images.
Real-world challenges
Several issues will have to be addressed before AI can be adopted broadly for ophthalmic purposes in LMICs. Some details are minor, such as training staff. For example, camera operators that worked with the Google team in Thailand would sometimes forget that the images needed to be taken in ambient light, and they positioned some cameras near large windows or in artificially lit rooms. To address this, the group put drapes around the cameras, and moved them closer to a light switch so operators would remember to turn off the lights off when capturing images.
More from Nature Outlooks
Another pain point has been slow or interrupted Internet connections — a frequent occurrence in LMICs — delaying the upload or receipt of results from cloud servers, on which most of the programs run. Reliance on the cloud also brings legal problems. In Tanzania, for example, it’s against the law for clinical data to be transferred outside the country without a data-transfer agreement, so the images can’t easily be processed by an AI system that is housed in another country.
Besides all these obstacles, however, sits another, very significant one: cost. So far, tests of AI screening in LMICs have been funded mainly as part of clinical trials, and there aren’t many data on what it will cost to provide this technology in the real world.
A few studies suggest that AI could be slightly cheaper than conventional screening for diabetic retinopathy. A study based in rural China calculated that AI screening costs $180.19 per person, compared with $215.05 per person for screening by an ophthalmologist6. And a study of a retinopathy-screening programme in Singapore found that a semi-automated model cost $62 per person per year — cheaper than both a physician-based approach ($77) and a fully autonomous AI ($66)7. But any cost difference varies with the accuracy of AI — false positives are costly — and the price of physician labour in different communities.
Although AI has a lot of potential for screening and diagnostics, it cannot reduce the burden of visual impairment in LMICs alone. “There are lots of other issues that need to be addressed as well,” Cleland says. Access to treatment will be crucial to making the most of whatever gains AI screening delivers. “Getting diagnosed doesn’t mean that a person isn’t going to lose vision,” Cleland says. But, he adds, “improving diagnostics will help”.