Cell culture
A375, B16-F10, MDA-MB-231, MCF7, PyMT99, H1792, H1693, H1299, DLD1, HCT116 and MIA PaCa-2 cells were purchased from ATCC. Mouse KP and KPK cells were developed by T. Papagiannakopoulos and KPC cells were a gift from D. Bar-Sagi. All cells were cultured in DMEM (Gibco 11965118) with 10% FBS unless otherwise noted, and grown in 37 °C, 5% CO2 humidified incubators. H1792, H1693 and H1299 cells were maintained in RPMI 1640 (Gibco 11875119) supplemented with 10% FBS. Cells were authenticated by the ATCC FTA Sample Collection Kit for Human and Mouse Cell Authentication Service and confirmed as mycoplasma-negative. Cultures were supplemented with GlutaMAX (Gibco 35050061), l-glutamine (Gibco 25030081). Amino acids l-leucine, l-serine, glycine, l-methionine, l-lysine and l-arginine were purchased from Sigma-Aldrich. We also supplemented cultures with Casitone (Gibco 225910) and Tryptone (Gibco 211705), both of which lack free glutamine51. Ala-Leu, Ala-Gly, Ala-Ser and all glutamine-containing dipeptides were purchased from GenScript or AnaSpec.
Cells were treated with bestatin (Cayman Chemicals 70520 or Sigma-Aldrich B8385), GPNA52 (l-γ-glutamyl-p-nitroanilide; Selleckchem S6670), BenSer53 (Sigma-Aldrich 13900), DAPT (Tocris 2634), Doxycycline (Tocris 4090), Linagliptin (Selleckchem S3031), anti-aminopeptidase N antibody (Millipore MABF2147), Cilastatin (SCBT sc-201312), JPM-OEt (Fisher, 502029515), Protease Inhibitor Cocktail (Sigma-Aldrich P8340), vacuolin-1 (Cayman Chemicals 20425), 5-(N-ethyl-N-isopropyl)amiloride (EIPA; Sigma-Aldrich A3085) and 3-methyladenine (3-MA; Selleckchem S2767).
Lysine[Z(NO2)]-valine (LNV) synthesis
The Fmoc-protected dipeptide was formed by first activating Fmoc-Lys(pNZ)-OH54 with EDC (3-(ethyliminomethyleneamino)-N,N-dimethylpropan-1-amine) to form the pentafluorophenolate ester. Addition of a solution of valine and sodium carbonate in water provided the dipeptide, which was purified on silica with dichloromethane and methanol. Finally, Fmoc was removed using piperidine in acetonitrile, then precipitated in cold ether. The solid was dissolved in 3:1 acetonitrile:water and lyophilized, giving a fluffy white powder. UV, mass spectrometry and 1H-NMR spectra are consistent with reported data.
High-throughput live microscopy
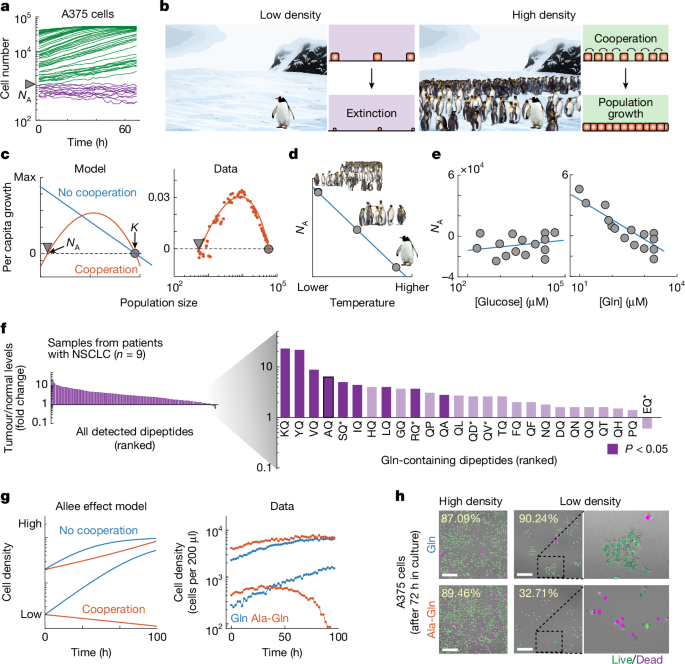
Cells were engineered to express nuclear localized fluorescent protein (H2B–YFP, Addgene #26000). Cells were seeded into 96-well culture plates at different densities and allowed to adhere overnight in glucose/glutamine-free DMEM (Gibco A1443001), 10% dialysed FBS (GeminiBio 100-108), 2 mM glutamine, 12.5 mM glucose (Sigma-Aldrich D9434) and antibiotic-antimycotic (Gibco 15240096). After 24 h, the plates were washed with PBS (Gibco 14040182) and medium was replaced with glucose/glutamine free DMEM, 1% dialysed FBS, 12.5 mM glucose, antibiotic-antimycotic and propidium iodide (MP Biomedicals 0219545810) to detect non-viable cells. These media were supplemented with 2 mM glutamine, 2 mM oligopeptides or neither. Population growth was tracked with an automated robotic microscope and incubator for up to 96 h (Cytation5/BioSpa8, BioTek). We used the same approach for Leu and Gly/Ser experiments but with DMEM containing Gln and missing these other amino acids.
Conditioned media experiments
Two million cells were plated in 10 cm dishes in glucose/glutamine-free DMEM, 10% dialysed FBS, 25 mM glucose, and 2 mM glutamine to adhere. The next day, the medium was removed, the plates were washed with PBS, and the medium replaced with glucose/Gln-free DMEM, 2 mM Ala-Gln, and 12.5 mM glucose and cells were grown for 24–72 h. After confirming that cell viability was above 90% (measured by Trypan Blue, Thermo Scientific), conditioned media were collected and filtered using 0.2-μm filters. When needed, conditioned media were fractioned by Amicon Ultra-15 Centrifugal Filter Unit with 3 kDa cutoff (Millipore UFC900308) and spun at 4,000g for 40 min. To clean high molecular mass fractions, the filter was rinsed with PBS and centrifuged at 4,000g, 40 min twice. Protein fractions were analysed by LC/MS by the proteomics core at NYU Langone School of Medicine.
For cell-free enzymatic activity, protein fractions were collected as described above in duplicates. Half of the volume for each sample, were heat-inactivated at 100 °C for 1 h. Then, all samples were transferred to a new Eppendorf tube supplemented with glucose/glutamine free DMEM, 12.5 mM glucose, 2 mM Ala-Gln or 2 mM Ala-Gln and 60 μM bestatin without cells and incubated overnight at 37 °C as well as 4 °C for control. After incubation, 2,000 cells per well in 96-well plate were treated in 100 μl cell-free enzymatic media mixed with 100 μl glucose/glutamine free DMEM, 2% dialysed FBS, 12.5 mM glucose, propidium iodide and grown for 3 days. The growth of cell populations was tracked by high-throughput live microscopy and image analysis were used to count live (YFP-labelled) and dead cells (propidium iodide-stained). For glutamine measurements, experiments were conducted in triplicate and were analysed by YSI 2950D-3 Biochemistry Analyzer.
Image analysis
Most of our in vitro experiments required tracking the growth of multiple cell populations using automated time-lapse imaging. This process generates many images—typically more than 20,000 per experiment—which required an efficient image analysis pipeline. To this end, we overhauled our image analysis algorithms20,55,56 and optimized to run on a graphics processing unit. In brief, our code uses natural intelligence and human learning to find, locate and count cells via the IDL Particle Tracking algorithm57. This algorithm uses a bandpass filter to remove the background noise and then employs a peak detection routine to rapidly locate cells without the need of image segmentation. To calculate the areas between cells, we used the Delaunay triangulation to find the closest neighbours of each cell. Then, the area of these triangles was calculated using the ‘area of polygon’ (polyarea) function in Matlab. Local cell densities are approximated as the inverse of the local area. To quantify levels of fluorescence, we first binarized grayscale nuclei images (typically stained with Hoechst) to create nuclear masks of individual cells. Masks of adjacent cells were separated by a watershed transformation using the peaks from the IDL algorithm as ‘catchment basins’. We then gently expanded these areas so they would also cover cytosolic areas of cells. Finally, we used these masks to obtain per cell properties such as location, area and pixel values from each fluorescent channel. All these analyses routines were written in Matlab. The scripts for our adaptation of the IDL Particle Tracking algorithm, including a graphical user interface for users unfamiliar with Matlab, are available at https://doi.org/10.5281/zenodo.14297705 (ref. 58).
Metabolic tracing
Extracts dried in an evaporator (Genevac EZ-2 Elite) were resuspended by incubating with shaking at 30 °C for 2 h in 50 μl of 40 mg ml−1 methoxyamine hydrochloride in pyridine. Metabolites were further derivatized with 80 μl of N-methyl-N-(trimethylsilyl) trifluoroacetamide + 1% TCMS (Thermo Fisher Scientific) and 70 μl ethyl acetate (Sigma-Aldrich) and then incubated at 37 °C for 30 min. Samples were analysed using an Agilent 7890 A Gas Chromatograph coupled to an Agilent 5977C mass selective detector. The gas chromatograph operated in splitless injection mode with constant helium gas flow at 1 ml min−1; 1 μl of derivatized metabolites was injected onto an HP-5ms column and the gas chromatograph oven temperature ramped from 60 to 290 °C over 25 min. Peaks representing compounds of interest were extracted and integrated using the MassHunter software v.B.08 (Agilent Technologies) and then normalized to both the internal standard (d-2-hydroxyglutaric-2,3,3,4,4-d5 acid (d5-2HG)) peak area and protein content of triplicate samples as determined by bicinchoninic acid assay (Thermo Fisher Scientific). The following ions were used for quantifying glutamine levels and 13C isotope distributions: glutamine, m/z 362–372; d5-2HG, m/z 354. Correction for natural isotope abundance was performed using IsoCor software v.1.0. All peaks were manually inspected and verified relative to known spectra for each metabolite. The concentration of glutamine in cell lysates, conditioned media and BALF was determined using a colorimetric Glutamine Assay Kit (Sigma-Aldrich MAK438) according to the manufacturer’s instructions.
Estimation of Gln consumption and Ala-Gln cleavage rates
We seeded 2 × 104 A375 cells expressing H2B-YFP in 96 well plates with Gln-U, Ala-Gln, or both. We then imaged these cells for 24 h every 6 h. After this process we rapidly collected their conditioned media. To obtain the levels per cell we integrated cell number according to: \([Q(N,t)]=\frac{1}{V}\frac{{Q}_{24}-{Q}_{0}}{{\int }_{0}^{t=24}N(t){\rm{d}}t}\), where \({Q}_{24}-{Q}_{0}\) correspond to the difference of initial and final concentrations of glutamine. The denominator corresponds to the total cell number integrated over time times the total volume (200 μl). Cell numbers were obtained using data from time lapse.
Synthesis and validation of PEGylated forms of bestatin
We decided to add PEG chains to the 5-carboxylate group of bestatin, which has been previously modified without interfering with its inhibitory activity59. In brief, diimide-activated Boc-leucine was coupled to PEG12-amine, followed by Boc removal with trifluoroacetic acid afforded the trifluoroacetic acid salt of the PEGylated leucine. This was coupled with a benzyloxycarbonyl (Cbz)-protected (2S,3R)-3-amino-2-hydroxy-4-phenylbutyric acid under non-racemizing conditions, using EDC and HOOBt (3-hydroxy-1,2,3-benzotriazin-4-one). After crude purification on silica, this mixture was acetylated with acetic anhydride in pyridine, which, after careful chromatography, provided the fully protected PEGylated bestatin. Hydrogenation followed by methanolysis to remove the Cbz and acetate, respectively, provided PEG-bestatin, which was purified on a low pressure C8 column, 30–70% MeCN/0.1% HCl (aq). To test these drugs, 20,000 lung KPK cells per well were seeded in 96-well plates and grown in glucose/glutamine free DMEM containing 12.5 mM glucose, propidium iodide (MP Biomedicals 0219545810) and 2 mM of either Gln or Ala-Gln. The 6 variants of PEGylated bestatin were added at different concentrations and cell growth was tracked over time as described before.
Animal work and tumour models
Female C57BL/6J mice (JAX 000664) around 11 weeks old were placed onto control (Envigo TD.110839) or serine/glycine-deficient diet (−Ser/Gly diet, Envigo TD.160752). After 4 days, mice were inoculated with 5 × 104 mouse lung wild-type or Cndp2-KO KP (KrasG12D; Trp53−/−) or KP Keap1-KO (KrasG12D; Trp53−/−; Keap1-KO) tumour cells in 100 μl of PBS (Gibco 14040182) intradermally, in the back and maintained in control or −Ser/Gly diet diet monitored daily. Next day, mice were started to be injected intraperitoneally with 250 mg kg−1 of bestatin-HCl (Cayman 70520), an equimolar PEGstatin solution (bestatin-PEG12, see above) or PBS every day for 10 days. Once tumours became palpable, measurements were performed with a calliper every 3 days and tumour volumes were calculated using the formula volume = (length × (width)2)/2. We considered mice free of tumours when tumours were undetectable or remained as small cysts (<20 mm3). Source data can be found in Supplementary Tables 5–10. Mice were euthanized by CO2 inhalation followed by cervical dislocation before tumours reached maximal permissible size (1,500 mm3 per tumour as defined by the Institutional Animal Care and Use Committee (IACUC)) or ulceration. Researchers were not blinded to the experimental groups during in vivo treatments and measurements. All mouse experiments were performed according to National Institutes of Health’s Guide and were approved by the New York University Animal Welfare Committee.
For in vivo competition experiments, mice were placed in the −Ser/Gly diet and after 4 days, they were inoculated with 1:1 mix of 2 × 104 mouse lung wild-type or Cndp2-KO KP tumour cells in 100 μl of PBS (Gibco 14040182) intradermally and maintained in the −Ser/Gly diet. Tumours were collected at different time points (before reaching the maximum size defined by IACUC) and frozen for cryosections or dissociated for flow cytometry. Dissociated tumours were fixed with Cyto-Fast Fix/Perm Solution (Biolegend 750000133) for 20 min, centrifuged, and the pellet was washed with PBS. Then per cell mCherry and YFP values were determined via flow cytometry (Cytek Aurora) and the data were analysed in FlowJo.
Orthotopic lung tumours
Cells derived from KrasG12D; Trp53−/− lung adenocarcinoma tumours, were engineered to express a dominant-negative Keap1 mutant (Keap1R470C)60. After continuous selection with 8 µg ml−1 puromycin for 5 days, the cells were incubated without puromycin selection media. Following an overnight incubation, cells were trypsinized and filtered twice through a 70-µm strainer and then resuspended in sterile PBS to a concentration of 500,000 cells per ml. C57BL/6J mice were then injected with 200 µl of this cell suspension via the tail vein using a 27G needle. Tumours were then embedded, sectioned, and processed for haematoxylin and eosin staining. Entire tumour sections were then imaged using an Aperio AT2 slide scanner (Leica). Tumour burden was calculated as the percentage of the lung area taken by tumours using the Q-Path software. For each mouse, we average the tumour burden from five sections.
Tumour collection and sectioning
Tumours were fixed with 4% paraformaldehyde (Thermo Scientific) in PBS at 4 °C, overnight. The next day, tumours were transferred into 30% sucrose in PBS solution and incubated at 4 °C until tumours were sunk onto the bottom of the 1.5 ml centrifuge tubes. Tumours were embedded into a cryostat embedding medium (Tissue-Tek O.C.T. Compound) at −20 °C. Sections were taken as 15–20 μm thickness onto polysine-coated slides (Thermo Scientific J2800AMNZ) using a cryostat (CM8150, Leica). A barrier around the tissue was drawn with a liquid blocker pen (ImmunoPen; Calbiochem) to prevent solution leakage. Permeabilization was performed by incubating the sections in 0.1% Tween. Then, the sections were blocked for 30 min in 2.5% bovine serum albumin (BSA). After blocking, the sections were incubated overnight with a primary antibody against CD31 to detect blood vessels (1:100, BD 550274). After washing the primary antibody with PBS, the sections were incubated with a secondary antibody (AlexaFluor 647; Thermo Fisher) diluted 1:1,000 in blocking solution for 1 h. For nuclear counterstaining, Hoechst 33342 (Thermo Fisher, H1399, 1 μg ml−1) was added to the same secondary solution. After PBS washes, the sections were mounted using Fluoromount-G (Thermo Fisher) and stored in the dark and imaged within 1 or 2 days. Keyence BZ-X810 microscope was programmed to image consecutive image fields. These fields were stitched together using the BZ-X800 Analyzer software. Final images were exported as raw 16-bit tiffs and analysed in Matlab.
Tumour organoid cultures
Tumours were excised from mice, immediately placed on DMEM with 10% FBS media in a 6-well plate, poked with a 25G needle syringe loaded with a protease mixture of 4 mg ml−1 collagenase IV, 2 U ml−1 dispase, and 2 U ml−1 DNase I (3 ml solution per tumour), and incubated 5 min at room temperature. Tumours were then cut into smaller chunks and incubated at 37 °C for 30 min. After incubation, two frosted glass slides were used to disrupt the tumours mechanically. Once all the tumour pieces were fully disrupted, cell suspension was filtered through 70-μm filters into a 50-ml Falcon tube and vortexed at high speed for 2–3 min. Then, the samples were centrifuged at 1,500 rpm for 10 min. The pellets were resuspended and seeded into 10 cm plates with DMEM, 10% FBS with penicillin–streptomycin. After 48 h, the cells were trypsinized and re-suspended in ice-cold glucose/glutamine free DMEM, 1% FBS, 12.5 mM glucose and 5% growth factor- reduced Matrigel (Corning 356230). Two thousand cells in 100 μl were plated per well in a 96-well plate already coated and solidified with 35 μl per well growth factor-reduced Matrigel. The plate was incubated 1 h in 37 °C incubator and then 100 μl glucose/glutamine free DMEM, 1% dialysed FBS, 12.5 mM glucose, 5% growth factor reduced Matrigel with 2 mM glutamine, 2 mM Ala-Gln, or no glutamine and 60 μM bestatin were added. Medium was changed to fresh every two days and the cells were grown for ten days. Imaging was carried out in All-in-One Fluorescence Microscope (BZ-X810 Keyence).
Generation of knockout cell lines by CRISPR–Cas9 gene editing
For Penta-KO cell lines, Anpep, Lnpep, Trhde and Rnpep single guide RNAs (sgRNAs) were first cloned into pX330 (Addgene 42230), lentiCRISPR-v2-neo (Addgene 98292), lentiCRISPR-v2-blasticidin (Addgene 98293), or lentiCRISPR-v2-hygromycin (Addgene 98291), respectively. These plasmids were linearized with BsmBI by T4 ligase (NEB). Then, sgRNAs targeting 12 different aminopeptidases were cloned individually into LentiCRISPR-v2-puro vector (Addgene 98290). The oligonucleotides used to generate sgRNAs were purchased from IDT and sequences are provided in Supplementary Table 2. For virus production, the sgRNA-expressing vector and lentiviral packaging vectors (VSV-G and Delta-8.9) were transfected into HEK 293T cells using Lipofectamine transfection reagent (Thermo Scientific L3000008). After 16–18 h, the transfection medium was replaced with fresh medium. Lentiviral supernatants were collected and filtered through a 0.45-μm filter 48 h after transfection. The supernatant was then mixed in a 1:1 ratio with fresh medium supplemented with polybrene (MilliporeSigma TR1003G) to a final concentration of 8 µg ml−1. This mixture was added to cells at 40–50% confluence in 6-well plates. After 48 h, transduced cells were transferred to a 10 cm plate followed by selection of desired antibiotics: puromycin (1 µg ml−1, Gibco A1113803) for 3–4 days, G418 (1 mg ml−1, Sigma-Aldrich A1720-1G) for 7 days, blasticidin (5 μg ml−1, InvivoGen ant-bl-05) for 5 days and hygromycin (0.5 mg ml−1, Invivogen ant-hg-1) for 7 days.
All Cndp2-KO cells were generated using the pX330 CRISPR vector. For point mutations, oligonucleotides for the sgRNA and the reverse complement sequences were synthesized and cloned into the pX330 vector. Cells were transfected with sgRNA-expressing vector using Lipofectamine. After 48 h, the cells were seeded as single cells per wells into 96 well plates. Cells were cultured as single clones for 2–3 weeks. Then, genomic DNA was isolated from each clone and the targeted region was amplified and analysed by DNA sequencing. Sequences of the primers used for this reaction are available in Supplementary Table 3. CNDP2 expression was also validated by immunoblotting. For Cndp2 deletion, sgRNAs targeting the beginning and the end of Cndp2 gene were designed following a previously described protocol61 and cloned individually into the pX330 vector. Cells were co-transfected with pX330-sgRNA1, pX330-sgRNA2 and a puromycin resistance gene containing vector. Cells were selected with puromycin for 4 days. Puromycin resistant cells were sorted into single cells to generate clones that were then screened for the deletion of Cndp2. The sgRNAs used for whole gene deletion are provided in Supplementary Table 2.
Identification of CNDP2
As peptidases may have overlapping and redundant functions, we used CRISPR–Cas9 to generate a tetra-KO line (TKO) for 4 of the 16 secreted aminopeptidases detected in conditioned media. These cells were knockout for Trhde, Anpep, Lnpep and Rnpep. We then knocked out each of the other 16 genes generating 12 penta-KO lines. Finally, we grew each of these lines in media with glutamine or with Ala-Gln and we compared their growth to parentals, TKO and bestatin-treated cells.
Cloning and testing of CNDP2 variants with signal peptides
To re-express CNDP2, we designed lentiviral vectors containing the mouse Cndp2 gene under the control of the CMV or PGK promoters and resistance to puromycin (lentiviral sequences can be found in Supplementary Table 1). For constructs with signal peptides, we introduced a 6×-His tag on the C terminus of CNDP2 and the nuclear localization signal from SV40 or the signal peptide from IL-2 as N-terminus signal peptides. All vectors were synthetized by VectorBuilder. CNDP2 levels and localization were validated by western blotting against CNDP2 or by immunofluorescence using an anti-His tag antibody (1:400, Invitrogen R930-25). To inhibit the secretory pathway, we incubated cells with Brefeldin A (1:1,000, Biolegend 420601) for 5 h.
Experiments with recombinant CNDP2
Twenty thousand lung KPK cells per well were seeded in 96-well plates and grown in glucose/glutamine-free DMEM containing 12.5 mM glucose, 2 mM Ala-Gln and propidium iodide (MP Biomedicals 0219545810). Recombinant CNDP2 protein (Sino Biological, Cat: 50246-M08B) was added to the media at different concentrations.
Testing intracellular roles of CNDP2
To test the contribution of other roles of CNDP2 including glutathione processing62,63,64,65, and in the formation of lactate-amino acid pseudopeptides66,67, we added either oxidized glutathione (GSSG; 2 mM, Sigma-Aldrich G6654), reduced glutathione (2 mM Sigma-Aldrich G6013) or N-lactoylphenylalanine (Lac-Phe; 2 mM Sigma-Aldrich SMB01375) to cultures of Cndp2-KO cells. None of these compounds were able to rescue the growth of Cndp2-KO cells.
To test whether CNDP2 modulated the PI3K–mTOR pathway68 in our system, we compared the levels of phospho-AKT, AKT, phospho-mTOR and mTOR in wild-type and Cndp2-KO cells by western blot. We did not observe any changes in the levels of these proteins when comparing wild-type and Cndp2-KO cells.
Western blotting
Protein lysates were collected in ice-cold RIPA buffer (0.05 M Tris-HCl pH 7.5, 0.15 M NaCl, 1% Triton-X 100, 1% sodium deoxycholate, 0.1% SDS) supplemented with freshly added protease inhibitors and centrifuged for 20 min at 14,000 rpm at 4 °C. For secreted proteins, the conditioned media were collected, and the proteins were concentrated using Amicon Ultra-15 Centrifugal Filter Unit with 3 kDa cutoff (Millipore Sigma UFC900308) by centrifuging for 1 h at 4,000g (4 °C) and obtained 200 μl of total sample. Protein concentrations of both lysates and concentrated conditioned media were measured by bicinchoninic acid (BCA) assay (Pierce, Thermo Scientific). Same amounts of protein were loaded on precast mini-PROTEAN TGX (Bio-Rad 4561095) gels followed by transfer to nitrocellulose membrane. Membranes were incubated with primary antibodies (CNDP2 1:1,000, Millipore Sigma HPA036898; CNDP2 1:500, Proteintech 14925-1-AP; actin 1:10,000, Cell Signaling 8H10D10; VDAC 1:1,000, Cell Signaling 4661; phospho-AKT 1:2,000, Cell Signaling 4060; AKT 1:2,000, Cell Signaling 2920; phospho-mTOR, 1:1,000, Cell Signaling 5536; mTOR 1:1,000, Cell Signaling 2983; α-tubulin 0.5 μg ml−1, Sigma-Aldrich T6199) overnight at 4 °C diluted in TBS-T supplemented with 5% BSA. Horseradish peroxidase (HRP)-linked secondary antibodies were used (HRP anti-mouse 1:10,000, Cell Signaling 7076; HRP anti-rabbit 1:8,000, Cell Signaling 7074). SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) or 1-Shot Digital ECL Solution (Kindlebio) was used for detection. Protein bands were imaged using a camera image detection system (KwikQuant).
Quantitative PCR with reverse transcription
Total RNA from a panel of human and mouse cancer cell lines was prepared using Trizol (Thermo Fisher Scientific) and reverse-transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The transcripts were quantified by quantitative PCR with Luminaris HiGreen qPCR Master Mix (Thermo Scientific) using a CFX Connect Real-Time PCR Detection System (Bio-Rad). Actin levels was used for normalization. Primer sequences are provided in Supplementary Table 4.
In vitro competition experiments
For in vitro competition experiments, lung KP Cndp2-WT cells were labelled with H2B–mCherry or with H2B–YFP and Cndp2-KO cells were labelled with H2B–YFP. Pairs of these cells were seeded in 96-well plates at a final concentration of 20,000 cells per well for all conditions. The initial ratios of cooperators:cooperators or cooperators:non-cooperators were 1:1, 1:10 and 1:100. Full DMEM containing Gln was used to seed the cells. Cells were grown overnight and then the full medium was replaced with medium containing either Gln or Ala-Gln. For short-term experiments, cells were grown for 4 days and imaged every 4 h. Population growth was tracked with an automated robotic microscope and incubator (BioTek Cytation 5 Imaging Reader/ BioSpa 8 Automated Incubator). For long-term experiments, cells were imaged once a day for 30 days. Whenever confluency was reached, cells were trypsinized and 10% of the cell suspension was re-seeded in full media containing Gln. The next day, the medium was replaced with medium containing either Gln or Ala-Gln. For both short- and long-term experiments, clones were identified and quantified using custom image analysis software.
Relative fitness of two cell clones designated the ratio of their respective fitness (W) calculated as the average number of cell divisions occurring over time, thus: \({W}_{i}={\log }_{2}({N}_{i,t}/{N}_{i,0})\), where N is the cell number of population i at time t.
MEMIC experiments
MEMICs were built as previously described20. Glass in MEMICs was coated with poly-d-lysine (Sigma-Aldrich, P6407). Lung KP Keap1 Cndp2-wild-type cells labelled with H2B–mCherry and Cndp2-KO cells labelled with H2B–YFP were seeded at a final concentration of 40,000 cells per chamber. For co-cultures of wild-type and knockout cells, the same total cell concentration was used and two initial cell ratios of 1:1 and 1:10 were used. Full medium containing glutamine was used to seed the cells. Cells were grown overnight and then the full medium was replaced for Ala-Gln media. The medium was changed for fresh medium every two days and the cells were grown for seven days. Chambers were imaged using an All-in-One Fluorescence Microscope (BZ-X810 Keyence) and analysed using custom image analysis software.
Statistics
We conducted at least three biological replicates of all experiments. Unless noted, we used two tailed Student’s t-tests to estimate P values between two conditions. In dot and line plots, error bars show s.d. from the mean. In box plots, centre lines show the median and box edges show 75th and 25th empirical quartiles of the data.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.