Cell culture
MOLM-13 (ATCC) and SET-2 (DSMZ) cells were a gift from M. D. Shair. HEK293T cells (Thermo Fisher Scientific) were a gift from B. E. Bernstein. Gesicle Producer 293T cells were a gift from D. R. Liu (Takara, 632617). MV4;11 and K562 cells were obtained from ATCC. HEK293F cells were obtained from Thermo Fisher Scientific. All mammalian cell lines were cultured in a humidified 5% CO2 incubator at 37 °C and routinely tested for mycoplasma (Sigma-Aldrich). RPMI1640 and DMEM media were supplemented with 100 U ml−1 penicillin and 100 µg ml−1 streptomycin (Gibco) and FBS (Peak Serum). MOLM-13, MV4;11 and K562 cells were cultured in RPMI1640 (Gibco) supplemented with 10% FBS. SET-2 cells were cultured in RPMI1640 (Gibco) supplemented with 20% FBS. HEK293T and Gesicle Producer 293T cells were cultured in DMEM (Gibco) supplemented with 10% FBS. HEK293F cells were cultured in Freestyle 293 Expression Medium (Thermo Fisher Scientific) shaking at 125 rpm. Spodoptera frugiperda (Sf9) insect cells (Expression Systems, 94-001F) were cultured in ESF921 medium (Expression Systems) in a non-humidified and non-CO2 incubator at 27 °C shaking at 140 rpm. High Five and ExpiSf9 cells were purchased from Thermo Fisher Scientific (B85502 and A35243, respectively), with Grace insect medium (Thermo Fisher Scientific, 11595030) supplemented with 10% FBS (Cytiva) and 1% penicillin–streptomycin (Gibco), cultured at 26 °C. All of the cell lines were authenticated by short tandem repeat profiling (Genetica) and routinely tested for mycoplasma (Sigma-Aldrich).
Lentiviral production
For lentivirus production, transfer plasmids were co-transfected with GAG/POL and VSVG plasmids into 293T cells using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. Medium was exchanged after 6 h and the viral supernatant was collected 52 h after transfection and sterile-filtered (0.45 µm). MOLM-13 and K562 cells were transduced by spinfection at 1,800g for 1.5 h at 37 °C with 5 µg ml−1 and 8 µg ml−1 polybrene (Santa Cruz Biotechnology), respectively. Where necessary, 48 h after transduction, cells were selected with 1 µg ml−1 and 2 µg ml−1 puromycin (Thermo Fisher Scientific), respectively, for 3–5 days. For inducible expression experiments, K562 cells were selected with or 600 µg ml−1 geneticin (G418 sulfate) (Thermo Fisher Scientific) for 7–10 days.
Plasmid construction
sgRNAs were ordered as synthetic oligonucleotides (Azenta/Genewiz), annealed and ligated into the appropriate vector: lentiCRISPR.v2 (Cas9 knockout), a gift from F. Zhang (Addgene, 52961); pRDA_478 (Addgene, 179096), which expresses BE3.9 (SpG), or pRDA_479 (Addgene, 179099), which expresses ABE8e (SpG) for base editing (gifts from J. Doench and D. Root). For individual sgRNA validation of the KBTBD4 CBE screen, sgRNAs were cloned into a pRDA_256 (Addgene, 158581) vector, a gift from J. Doench and D. Root, containing SpG Cas9 NG PAM. Other plasmids were cloned by Gibson Assembly using NEBuilder HiFi (New England Biolabs). Cloning strains used were NEB Stable (lentiviral) and NEB 5-alpha (other plasmids) (New England Biolabs). For base editor cloning, bacterial cultures were grown at 30 °C. Final constructs were validated by Sanger sequencing (Azenta/Genewiz).
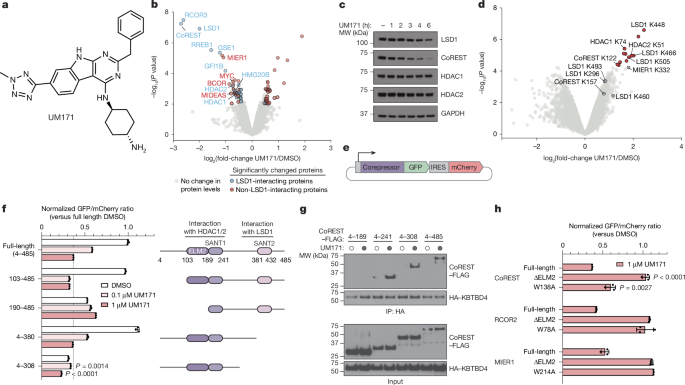
All KBTBD4 expression plasmids encoded isoform 1 (human, residues 1–518) but longer isoform 2 (residues 1–534) numbering was used. CoREST expression plasmids encoded isoform 1 (human) in either full-length (considered residues 4–485) or various truncations. Open reading frames (ORFs) of human KBTBD4 and RCOR1 (mammalian expression) were obtained from Horizon Discovery. The full-length MIER1 isoform 1 (human, residues 1–512) ORF was obtained from GeneCopoeia and full-length RCOR2 isoform 1 (human, residues 1–523) was a gift from M. L. Suvà. The LSD1 ORF was a gift from R. Shiekhattar. Full-length HDAC1 ORF was a gift from E. Verdin (Addgene, 13820). The coding sequence of HDAC2 (amino acids 2–488) was synthesized by IDT. The coding sequence of full-length NUDCD3 (human, residues 1–361) was synthesized by Twist Biosciences.
For fluorescent and stability reporter constructs, CoREST, MIER1, RCOR2 and KBTBD4 were cloned into Cilantro 2, a gift from B. Ebert (Addgene, 74450). For transfection constructs, CoREST–FLAG and HA–KBTBD4 constructs were cloned into pcDNA3. For KBTBD4 overexpression constructs, KBTBD4 coding sequences were cloned into pSMAL mCherry, which was generated from pSMAL through introduction of an mCherry ORF into pSMAL (a gift from J. E. Dick), or pFUGW-IRES-puro, which was generated from pFUGW (Addgene, 14883) by replacing the UbC promoter-eGFP cassette with an EFS-NS-IRES-puromycin cassette. For inducible expression constructs, KBTBD4 coding sequence (CDS) was cloned into pInducer20, a gift from S. Elledge (Addgene, 44012). For bacmid expression, KBTBD4 and NUDCD3 were cloned into pFastbac, a gift from T. Cech. The KBTBD4 construct for structure determination was made by cloning human KBTBD4 cDNA isoform 1 into a pFastBac vector with a tandem 10×His-tag and MBP tag at the N terminus followed by a TEV protease cutting site. For eVLP constructs, sgRNA sequences were cloned into pU6-sgRNA (a gift from D. R. Liu) by PCR amplification, and co-transfected with pCMV-MMLVgag-3×NES-ABE8e (Addgene, 181751), pBS-CMV-gagpol (Addgene, 35614) and pCMV-VSV-G (Addgene no. 8454), gifts from D. R. Liu, P. Salmon, and B. Weinberg, respectively.
CRISPR–Cas9-mediated genome editing
Knock-in of CoREST–GFP in K562 cells
mEGFP followed by a ‘GGGSGGGS’ linker was knocked into the C terminus of CoREST in K562 cells. sgRNA (sgRNA: TTCAAAGCCACCAGTTTCTC) targeting the C terminus of CoREST was cloned into a Cas9 plasmid, PX45953, and electroporated according to the manufacturer’s protocol (Neon Transfection System, Thermo Fisher Scientific) with a repair vector containing the mEGFP CDS and linker flanked by 750 bp of genomic homology sequences to either side of the CoREST C terminus. In brief, 2 × 105 cells were washed twice with PBS and resuspended in buffer R. PX459 (0.5 µg) and the repair vector (0.5 µg) were added to the cell suspension, and electroporated at 1350 V with a 10 ms pulse width for 4 pulses using the Neon Transfection System 10 µl kit. After electroporation, cells were immediately transferred to prewarmed medium. To generate single-cell clones, cells were gated to sort for the top 0.2% GFP+ cells and single-cell sorted using the MoFlo Astrios EQ Cell Sorter (Beckman Coulter), expanded and validated by western blotting and Sanger sequencing.
Knock-in of HDAC2–dTAG in HDAC1-null CoREST–GFP K562 cells
Homology-directed repair was used to insert a linker-FKBP12F36V-2xHA-P2A-PuroR cassette into the C terminus of HDAC2 in HDAC1-null CoREST–GFP K562 cells (generation described below). sgRNA (sgRNA: GGTGAGACTGTCAAATTCAG) (Synthego) targeting the C terminus of HDAC2 was electroporated according to the manufacturer’s protocol (Neon Transfection System, Thermo Fisher Scientific) with a repair vector containing the linker-FKBP12F36V-2×HA-P2A-PuroR CDS flanked by 700–800 bp of genomic homology sequences to either side of the HDAC2 C terminus. In brief, 2 × 106 cells were washed twice with PBS and resuspended in buffer R. The sgRNA and the repair vector (0.5 µg) were added to the cell suspension, and electroporated at 1,350 V with a 10 ms pulse width for three pulses using the Neon Transfection System 100 µl kit. After electroporation, cells were immediately transferred to prewarmed medium. After 9 days of recovery, cells were selected with 2 µg ml−1 puromycin (Thermo Fisher Scientific) for 10 days before single-cell sorting on the MoFlo Astrios EQ Cell Sorter (Beckman Coulter). Single-cell clones were validated by Sanger sequencing and western blotting.
Generation of knockout K562 cells
Lentiviral vectors carrying sgRNA (LSD1, HDAC1, HDAC2) were generated by cloning appropriate sequences (LSD1: TAGGGCAAGCTACCTTGTTA; HDAC1: GCACCGGGCAACGTTACGAA; HDAC2: TACAACAGATCGTGTAATGA) into the pLentiCRISPR.v2 lentiviral vector. The control vector contained sgRNA targeting luciferase (sgControl). Lentivirus was produced and K562 CoREST–GFP cells were transduced and puromycin-selected as described above.
HDAC1-null, HDAC2-null and KBTBD4-null CoREST–GFP K562 clones were generated using the Alt-R CRISPR–Cas9 System (IDT) to deliver ribonucleoprotein complexes containing KO guides (HDAC1: GCACCGGGCAACGTTACGAA; HDAC2: TACAACAGATCGTGTAATGA; KBTBD4: GATATCTGTGAGTAAGCGGT) using the Neon Transfection System (Thermo Fisher Scientific) according to the manufacturer’s protocol. Transfected cells recovered for 72 h before sorting for single-cell clones on the MoFlo Astrios EQ Cell Sorter (Beckman Coulter). Single-cell clones were validated by genotyping and immunoblotting. sgRNA and primer sequences for validation are provided in Supplementary Tables 1 and 2, respectively.
Proteomics sample preparation
MV4;11 and SET-2 (50 million cells per replicate) were treated with 1 µM UM171 or DMSO for 6 h. Cells were washed twice with ice-cold PBS and snap-frozen in liquid nitrogen for storage at −80 °C until use (n = 3, biological replicates). Frozen cell pellets were lysed in DPBS (Thermo Fisher Scientific) supplemented with benzonase (Santacruz Biotechnology) and protease inhibitor cocktail (Roche) using a chilled bath sonicator at 4 °C (Q700, QSonica). The lysates were clarified by centrifugation at 300g for 3 min. Proteins were quantified by BCA assay (Thermo Fisher Scientific) and normalized to 200 µg per 150 µl. Then, 200 µg of protein was reduced with 5 mM Tris(2-carboxyethyl) phosphine hydrochloride (TCEP) (Sigma-Aldrich) for 2 min and alkylated with 20 mM chloroacetamide (CAA) for 30 min at room temperature. Next, 1,000 µg of magnetic SP3 beads (1:1 hydrophobic:hydrophilic) (Cytiva) was added to each sample along with 100% liquid chromatography (LC)–MS-grade ethanol (Sigma-Aldrich) to reach the final concentration of 50% ethanol. The samples were then incubated for 30 min with KingFisher Flex system (Thermo Fisher Scientific) at room temperature. The beads were washed three times with 80% high-performance LC (HPLC)-grade ethanol (Sigma-Aldrich) and resuspended with 150 µl of trypsin/Lys-C (4 µg, Thermo Fisher Scientific) in 200 mM EPPS (pH 8.4)/5 mM CaCl2 (Sigma-Aldrich), and proteins were digested overnight for 16 h at 37 °C. Digested peptides were dried by a Speedvac, reconstituted with 5% acetonitrile (Sigma-Aldrich)/0.1% formic acid (Thermo Fisher Scientific) and desalted using Empore C18 Extraction Disks (3 M). Peptides were eluted with 80% acetonitrile/0.1% formic acid, dried by a Speedvac. Peptides reconstituted with 5% acetonitrile/0.1% formic acid were quantified using Quantitative Colorimetric Peptide Assay (Thermo Fisher Scientific) and 10 µg of peptides for each sample were labelled with 50 µg of TMTpro16-plex reagents (Thermo Fisher Scientific) per channel. TMT labelling was performed for 75 min with rotation at room temperature, and reaction was quenched by adding 5% hydroxylamine (Acros Organics) for 15 min, followed by addition of 10% formic acid. The samples were then pooled and dried using a Speedvac.
High-pH reversed-phase peptide fractionation
Peptides were reconstituted with 300 µl of 5% acetonitrile/0.1% formic acid. Fractionation was performed using the Pierce High pH Reversed-Phase Peptide Fractionation Kit (Thermo Fisher Scientific) according to the manufacturer’s instruction. In brief, peptide samples were fractionated with 21 increments (7.5–55% with every 2.5% increase, and 75%) of acetonitrile with 10 mM NH4HCO3. Three eluents from every seventh fraction were pooled to get total seven fractions and dried using a Speedvac.
MS data acquisition
Fractionated samples were reconstituted with 2% acetonitrile/0.1% formic acid and analysed on the EASY-nLC 1200 system (Thermo Fisher Scientific) coupled to the Orbitrap Eclipse Tribrid Mass Spectrometer (Thermo Fisher Scientific) with the FAIMSpro system equipped with real-time search function. Peptides were loaded onto a trap column (Pepmap 100 C18, 3 μm particle size, 100 Å pore size, 75 μm inner diameter × 150 mm length) and separated over a 140 min gradient of 5–35% acetonitrile in 0.1% formic acid and a flow rate of 300 nl min−1 with an analytical column (EASY-Spray C18 HPLC, 2 μm particle size, 75 µm inner diameter × 500 mm length). Peptides were acquired by data-dependent acquisition (DDA) and quantified using synchronous precursor selection MS3 (DDA-SPS-MS3); In brief, peptides were ionized at 2,300 V, separated by FAIMSpro (1.5 s per cycle) and scanned for MS1 analysis (resolution of 120,000; scan range of 400–1,400 m/z; maximum ion injection time (IIT) 50 ms; automatic gain control (AGC) setting of 10,000). MS2 analysis was collected from collision-induced dissociation (collision energy of 36%), and MS3 spectra were analysed in the orbitrap (resolution, 50,000; mass range, 100–500 Da).
MS data analysis
Data processing was performed in ProteomeDiscoverer (PD) v.2.5 (Thermo Fisher Scientific) using the SequestHT algorithm. All raw files were submitted to search against the UniProtKB human universal database (UniProt: UP000005640, downloaded May 2020) combined with the common Repository of Adventitious Proteins (cRAP, classes 1, 2, 3 and 5) and the following parameters54; precursor tolerance of 10 ppm, fragment ion tolerance of 0.6 Da, minimum peptide length of 6 and trypsin full digestion with zero miscleavages. Cysteine carbamidomethylation (+57.021 Da) and methionine oxidation (+15.995 Da) were set as variable modifications while lysine- and N-terminus-TMTpro modification (+304.207 Da) were set as static modifications. Peptide-spectrum matches were filtered to a 1% false-discovery rate (FDR) using the Percolator algorithm (v.3.05.0) and further for protein assignment. Reporter ion quantifier node was set with the co-isolation threshold of 50, signal-to-noise threshold of 10 and SPS mass matches threshold of 50. Peptide abundance was normalized to total peptides. The protein ratio was calculated using the PD2.5 pairwise ratio-based algorithm and an empirical Bayes-moderated t-test was used to compare treatment groups using the limma R package (v.3.54.2)55. The R environment used was v.4.2.2. Data are provided in Supplementary Data 1 and 2. Volcano plots were created using the R package ggplot2 (v.3.5.1). Protein–protein interaction networks were constructed using STRINGdb (v.12)56, with a confidence threshold of >0.7, and the resulting networks were imported and visualized using Cytoscape (v.3.9.0).
Immunoblotting
Cells were lysed on ice in RIPA buffer (Boston BioProducts) with 1× Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific) and 5 mM EDTA (Thermo Fisher Scientific). The lysates were clarified by centrifugation and the total protein concentration was measured using the BCA Protein Assay (Thermo Fisher Scientific). The samples were electrophoresed and transferred to a 0.45 μm nitrocellulose membrane (Bio-Rad). The membranes were blocked with Tris-buffered saline Tween (TBST) with 5% blotting-grade blocker (Bio-Rad) and incubated with primary antibodies at the following dilutions: KBTBD4 (Novus Biologicals, NBP1-88587, 1:1,000), HDAC1 (Cell Signaling Technology, 34589, D5C6U, 1:1,000), HDAC2 (Cell Signaling Technology, 57156, D6S5P, 1:1,000), FLAG (Sigma-Aldrich, F1804, M2, 1:2,000), HA tag (Cell Signaling Technology, 3724, C29F4, 1:1,000), GAPDH (Santa Cruz Biotechnology, sc-47724, 0411, 1:10,000), H3K9ac (Abcam, AB32129, 1:2,000), H3 (Abcam, AB1791, 1:2,000). The membranes were washed three times with TBST and incubated with secondary antibodies at the following dilutions: anti-rabbit IgG HRP conjugate (Promega, W4011, 1:20,000), anti-mouse IgG HRP conjugate (Promega, W4021, 1:40,000) and goat anti-rabbit IgG HRP conjugate (Cell Signaling Technology, 7074, 1:2,000). After three washes with TBST, immunoblots were visualized using SuperSignal West Pico PLUS or SuperSignal West Femto chemiluminescent substrates (Thermo Fisher Scientific).
Ubiquitin and serial proteome
Sample preparation
SET-2 cells (10 million cells per replicate) were pretreated with 100 nM bortezomib or DMSO for 3 h and subsequently treated with 1 µM UM171 for 1.5 h or 6 h or DMSO for 6 h. Cells were washed twice with ice-cold PBS, and snap-frozen in liquid nitrogen for storage at −80 °C until use (n = 3, biological replicates). The samples underwent denaturing lysis in SDS to prepare for S-Trap digestion and lysed in 500 µl SDS lysis buffer (5% SDS, 50 mM TEAB pH 8.5, 2 mM MgCl2, 2 µg ml−1 aprotinin, 10 µg ml−1 leupeptin, 1 mM PMSF, 50 µM PR-619 (Lifesensors, SI9619: PR-619) and 1 mM chloroacetamide. The samples were disrupted by gentle vortexing and incubated at room temperature for about 15 min. The samples were treated with 3 µl 250 U μl−1 benzonase (Thomas Scientific, E1014-25KU) to shear DNA, mixed again and incubated at room temperature for another ~15 min. The lysates were cleared by centrifugation for 10 min at 20,000g and the supernatant was prepared for S-Trap digestion. The protein concentration was estimated using the BCA protein assay. Disulfide bonds were reduced in 5 mM DTT for 1 h at 25 °C and 1,000 rpm shaking, and cysteine residues were alkylated in 10 mM IAA in the dark for 45 min at 25 °C under 1,000 rpm shaking. Then, 12% phosphoric acid was added at a 1:10 ratio of lysate volume to acidify, and proteins were precipitated with 6× sample volume of ice-cold S-Trap buffer (90% methanol, 100 mM TEAB). The precipitate was transferred in successive loads of 3 ml to a S-Trap Midi (Protifi) and loaded with 1 min centrifugation at 4,000g, mixing the remaining precipitate thoroughly between transfers. The precipitated proteins were washed four times with 3 ml S-Trap buffer at 4,000g for 1 min. To digest the deposited protein material, 350 µl digestion buffer (50 mM TEAB) containing both trypsin and LysC, each at 1:50 enzyme:substrate weight:weight ratio, was passed through each S-Trap column with 1 min centrifugation at 4,000g. The digestion buffer was then added back atop the S-Trap and the cartridges were left capped overnight at 25 °C. Peptide digests were eluted from the S-Trap, first with 500 µl 50 mM TEAB and next with 500 µl 0.1% formic acid, each for 30 s at 1,000g. The final elution of 500 µl 50% acetonitrile/0.1% formic acid was centrifuged for 1 min at 4,000g to clear the cartridge. Eluates were frozen and dried in a vacuum centrifuge. Peptides were reconstituted in 30% acetonitrile/0.1% formic acid, and the concentration was estimated using the BCA assay.
Enrichment of K-ε-GG peptides
Enrichment of K-ε-GG peptides was performed using the UbiFast method as previously described57,58. For each sample, 500 µg peptides was reconstituted in 250 µl HS bind buffer (Cell Signaling Technology) with 0.01% CHAPS. Reconstituted peptide was added to 5 µl PBS-washed HS anti-K-ε-GG antibody bead slurry (Cell Signaling Technology, 59322) in a 96-well KingFisher plate (Thermo Fisher Scientific). The plate was covered with foil and incubated for 1 h at 4 °C with end-over-end rotation. The plate containing peptides and anti-K-ε-GG antibody beads was then processed on the KingFisher Flex as previously described57. In brief, bead-bound enriched peptides were washed with 50% acetonitrile/50% HS wash buffer followed by awash in PBS. K-ε-GG peptides were labelled while on-bead with freshly prepared 400 µg TMTpro reagents (Thermo Fisher Scientific) in 100 mM HEPES for 20 min and labelling was quenched with 2% hydroxylamine. The beads were then washed with HS wash buffer before being deposited into 100 µl PBS. All sample wells were combined, the supernatant was removed and enriched K-ε-GG peptides were eluted from the beads with 2 × 10 min 0.15% TFA. The eluate was desalted using C18 StageTips, frozen and dried in a vacuum centrifuge.
TMT labelling of UbiFast flow-through for serial proteome
Non-TMT-labelled K-ε-GG-enrichment flowthroughs were processed for proteome analysis as previously described59. In brief, peptides were acidified to 1% formic acid and desalted with 50 mg tC18 SepPak cartridges. The eluates were frozen and dried in a vacuum centrifuge. Peptides were reconstituted in 30% acetonitrile/0.1% formic acid, and the concentration was estimated using the BCA assay; 100 µg of each sample was reconstituted in 60 µl 50 mM HEPES and labelled with 200 µg TMTPro18 reagents at a final concentration of 20% acetonitrile for 1 h at 25 °C and 1,000 rpm. Labelling reactions were diluted to 5 mg ml−1 with 50 mM HEPES. Complete labelling and balancing of input material were confirmed. TMT labelling was quenched with 3 µl 5% hydroxylamine for 15 min and each TMTPro18 plex was combined, frozen and dried. Dried, labelled and combined peptides were reconstituted with 1 ml 1% formic acid and desalted with a 100 mg tC18 SepPak. The eluate was snap-frozen and dried in a vacuum centrifuge.
Offline bRP fractionation was performed to separate peptides over a 96 min gradient with a flow rate of 1 ml min−1. Solvent A was 5 mM ammonium formate/2% acetonitrile and solvent B was 5 mM ammonium formate/90% acetonitrile. In total, 96 fractions were concatenated into 24 fractions for proteome analysis. Then, 5 µg of peptides from each of the 24 fractions was transferred into HPLC vials, frozen and dried in a vacuum centrifuge for analysis. Proteome fractions were reconstituted in 3% acetonitrile/0.1% formic acid and 1 µg from each of the 24 fractions was injected for LC–MS/MS analysis.
LC–MS/MS for ubiquitin proteomics and serial proteome
K-ε-GG peptides were reconstituted in 9 µl 3% acetonitrile/0.1% formic acid and 4 µl was injected twice onto a Orbitrap Exploris 480 mass spectrometer coupled to the Vanquish Neo UHPLC system (Thermo Fisher Scientific), equipped with FAIMS (Thermo Fisher Scientific) essentially as previously described58. The sample was injected onto a capillary column (Picofrit with 10 µm tip opening/75 µm diameter, New Objective, PF360-75-10-N-5) packed in-house with approximately 25 cm C18 silica material (1.5 µm ReproSil-Pur C18, Dr. Maisch) and heated to 50 °C. Peptides were separated at a flow rate of 200 nl min−1 with a linear 154 min gradient from 1.8% solvent B (acetonitrile, 0.1% formic acid), 2 min 5.4% B, 122 min 31.5% B, 130 min 54% B, 133 min 72% B, 144 min 45% B, 149 min 45% B. MS1 spectra were measured with a resolution of 60,000, an AGC target of 100% and a mass range from 350 to 1,800 m/z. Up to 10 MS2 spectra per duty cycle were triggered at a resolution of 45,000, an AGC target of 50%, an isolation window of 0.7 m/z and a normalized collision energy of 32. The FAIMS device was operated in standard resolution mode using the compensation voltages of −40, −60 and −80 for the first injection followed by a second injection with compensation voltages of −45, −50 and −70.
Proteome fractions were reconstituted in 3% acetonitrile/0.1% formic acid, and 1 µg from each of the 24 fractions was injected for LC–MS/MS analysis onto an Orbitrap Exploris 480 mass spectrometer coupled to a Vanquish Neo UHPLC system (Thermo Fisher Scientific) essentially as previously described58. The sample was injected onto a capillary column (Picofrit with 10 µm tip opening/75 µm diameter, New Objective, PF360-75-10-N-5) packed in-house with approximately 30 cm C18 silica material (1.5 µm ReproSil-Pur C18, Dr. Maisch) and heated to 50 °C. Peptides were eluted into the Orbitrap Exploris 480 at a flow rate of 200 nl min−1. The bRP fractions were run on a 110 min method, including a linear 84 min gradient from 94.6% solvent A (0.1% formic acid) to 27% solvent B (99.9% acetonitrile, 0.1% formic acid), followed by a linear 9 min gradient from 27% solvent B to 54% solvent B. MS was conducted using a data-dependent acquisition mode, where MS1 spectra were measured with a resolution of 60,000, a normalized AGC target of 300% and a mass range from 350 to 1,800 m/z. MS2 spectra were acquired for the top 20 most abundant ions per cycle at a resolution of 45,000, an AGC target of 30%, an isolation window of 0.7 m/z and a normalized collision energy of 34. The dynamic exclusion time was set to 20 s, and the peptide match and isotope exclusion functions were enabled.
Data analysis for ubiquitin proteomics and serial proteome
MS data were processed using Spectrum Mill Rev BI.07.11.216 (https://proteomics.broadinstitute.org). Extraction of raw files retained spectra within a precursor mass range of 600 to 6,000 Da and a minimum MS1 signal-to-noise ratio of 25. MS1 spectra within a retention time range of ±45 s, or within a precursor m/z tolerance of ±1.4 m/z, were merged. MS/MS searching was performed against a human UniProt database. Digestion parameters were set to ‘trypsin allow P’ with an allowance of 4 missed cleavages. The K-ε-GG MS/MS search included fixed modifications, carbamidomethylation on cysteine and TMTPro on the N terminus and internal lysine, and variable modifications, acetylation of the protein N terminus, oxidation of methionine and K-ε-GG on tryptic peptide—‘Ubiquitin Residual GG from Tryp Cut on K’. The proteome MS/MS search included fixed modifications, carbamidomethylation on cysteine and TMTPro on the N terminus and internal lysine, and variable modifications, acetylation of the protein N terminus, oxidation of methionine, N-term deamidation, and N-term Q-pyroglutamate formation. Restrictions for matching included a minimum matched peak intensity of 40% for K-ε-GlyGly and 30% for proteome, and a precursor and product mass tolerance of ±20 ppm. Peptide-spectrum matches were validated using a maximum FDR threshold of 1.2% for precursor charge range to 2 to 6. A target protein score of 0 was applied during protein polishing autovalidation for the proteome to further filter peptide-spectrum matches. TMTpro reporter ion intensities were corrected for isotopic impurities using the afRICA correction method in the Spectrum Mill protein/peptide summary module, which uses determinant calculations according to Cramer’s rule. Protein quantification and statistical analysis were performed using the Proteomics Toolset for Integrative Data Analysis (Protigy, v.1.0.7, Broad Institute, https://github.com/broadinstitute/protigy). Each K-ε-GG peptide or protein was associated with a log2-transformed expression ratio for every sample condition over the median of all sample conditions. Median normalization was conducted separately on the K-ε-GG peptide data and the global proteome data. K-ε-GG peptide data were then normalized to the global proteome data using the panoply_ptm_normalization module of PANOPLY (PANOPLY, Broad Institute, https://github.com/broadinstitute/PANOPLY/wiki). Specifically, it takes all K-ε-GG peptide log-ratios in all samples and regresses them against the log-ratios of cognate proteins. Then, the resulting residuals are the normalized K-ε-GG peptide values. After normalization, an empirical Bayes-moderated t-test was used to compare treatment groups, using the limma R package55. P values associated with every modified peptide or protein were adjusted using the Benjamini–Hochberg FDR approach. Data are provided in Supplementary Data 4–6.
Fluorescence degradation reporter assay
CoREST (full-length and truncated), MIER1 and RCOR2 inserts were PCR-amplified with Esp3I sites and ligated into a Cilantro 2 eGFP-IRES-mCherry reporter vector by golden-gate assembly. Point mutations were introduced into coding regions using standard PCR-based site-directed mutagenesis techniques. Deletion constructs were made by PCR amplification of the appropriate regions and cloned into the Cilantro 2 vector using Gibson cloning (New England Biolabs). Lentiviral particles carrying the respective constructs in the Cilantro 2 vector were produced and used to transduce MOLM-13 cells as described above. Then, 48 h after transduction, cells were selected with 1 µg ml−1 puromycin for 3–5 days. The selected cells were then treated with various concentrations of UM171 or 0.1% DMSO for 6 or 24 h. GFP and mCherry fluorescence were measured on a NovoCyte 3000RYB flow cytometer (Agilent) after drug or DMSO treatment. The geometric mean of the ratio of GFP to mCherry fluorescence was calculated for each sample using the NovoExpress software (v.1.5.0, Agilent). The ratios for the individual drug-treated samples were normalized to the ratios of the DMSO-treated samples in Microsoft Excel (v.16.80) and plotted using GraphPad Prism (v.9.4.0). All degradation assays were done in triplicate and FACS-gating schemes are shown in Supplementary Fig. 1a.
Co-IP analysis
In K562 cells
FLAG–KBTBD4 was cloned into pFUGW-IRES-puro and stably expressed in CoREST–GFP K562 cells by lentiviral transduction followed by puromycin selection, as described above. Cells were pretreated with either 10 µM SAHA (1 h) or DMSO, then treated with 1 µM MLN4924 for 3 h then 5 µM UM171 or DMSO for 1 h. Cells were washed twice with cold PBS and flash-frozen. Co-IP was performed as described below.
In HEK293T cells
HEK293T cells were transfected with 3 µg pcDNA3.1 HA–KBTBD4 plasmid and 3 µg pcDNA3 CoREST–FLAG (full-length or truncated) using PEI MAX transfection reagent (Polysciences) according to the manufacturer’s protocol. Then, 48 h after transfection, cells were treated with 1 µM MLN4924 for 3 h then 1 µM UM171 or DMSO for 1 h. Cells were washed twice with cold PBS and flash-frozen. Co-IP was performed as described below.
Cells were thawed, lysed on ice in lysis buffer (25 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40 alternative) supplemented with cOmplete, EDTA-free protease inhibitor cocktail (Sigma-Aldrich) and the lysates were cleared. The protein concentration was quantified as described above and diluted to 1 mg ml−1 in lysis buffer with 1 µM UM171 or DMSO. The supernatants were immunoprecipitated overnight at 4 °C with 25 µl Pierce anti-HA magnetic beads (Thermo Fisher Scientific). The beads were washed six times with lysis buffer, eluted in SDS–PAGE loading buffer and carried forward to immunoblotting as described above.
Protein expression and purifications
Recombinant human KBTBD4 for biochemical and biophysical analyses was purified from Sf9 insect cells. cDNAs for human KBTBD4 and NUDCD3 proteins were cloned into the pFastBac donor vector and the recombinant baculoviruses were constructed using the Bac-to-Bac protocol and reagents (Thermo Fisher Scientific). KBTBD4 constructs were tagged on the N terminus with 6×His cleavable by TEV protease. These plasmids were used to prepare separate baculoviruses according to standard protocols (Bac-to-Bac Baculovirus Expression System, Thermo Fisher Scientific). Detection of gp64 was used to determine the baculovirus titre (Expression Systems). For expression, Sf9 cells were grown to a density of 1–2 × 106 cells per ml and co-infected with NUDCD3 baculovirus at a multiplicity of infection (MOI) of 2 and KBTBD4 baculovirus at a MOI of 3.5. The cells were incubated for 72 h (27 °C, 120g), collected and then frozen with liquid nitrogen for future purification. Cells were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.0 cold, 500 mM NaCl, 1 mM TCEP, 10% glycerol, 15 mM imidazole) supplemented with 1% NP-40, 1 mM PMSF and cOmplete, EDTA-free protease inhibitor cocktail (Sigma-Aldrich) and sonicated. The lysates were clarified by centrifugation at 100,000g for 30 min and incubated with His60 Ni Superflow affinity resin (Takara). Resin was washed with lysis buffer containing a stepwise gradient of 15–50 mM imidazole, followed by elution using lysis buffer with 250 mM imidazole. The eluate was exchanged into storage buffer (50 mM Tris-HCl, pH 8.0 cold, 150 mM NaCl, 1 mM TCEP, 10% glycerol) using an Econo-Pac 10DG desalting column (Bio-Rad) and further purified by size-exclusion chromatography using the Superdex 200 10/300 GL column (GE Healthcare). The purity of the recombinant protein was verified by SDS–PAGE and fractions with 90–95% purity were pooled and stored at −80 °C.
Recombinant human KBTBD4 used in cryo-EM structure determination was purified from Trichoplusia ni High Five insect cells. cDNAs for human KBTBD4 and NUDCD3 proteins were cloned into the pFastBac donor vector and the recombinant baculoviruses were constructed using the Bac-to-Bac protocol and reagents (Thermo Fisher Scientific). KBTBD4 constructs were tagged on the N terminus with 10×His and MBP tag cleavable by TEV protease. These plasmids were used to prepare separate baculoviruses according to standard protocols (Bac-to-Bac Baculovirus Expression System, Thermo Fisher Scientific). For expression, the monolayer High Five cells were grown to about 80% confluency and co-infected with NUDCD3 baculovirus. The cells were incubated for 72 h (26 °C), collected and then frozen with liquid nitrogen for future purification. Cells were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.0 cold, 150 mM NaCl, 1 mM TCEP) supplemented with 1 mM PMSF, 10 µM leupeptin, 0.5 µM aproptinin and 1 µM pepstatin A and sonicated. The lysates were clarified by centrifugation at 100,000g for 30 min and incubated with amylose affinity resin (New England BioLabs). Resin was washed with lysis buffer, followed by elution using lysis buffer with 10 mM maltose. The eluate was cut with tobacco etch virus protease overnight, followed by the prepacked anion-exchange column (GE Healthcare) to get rid of the protease and further purified by size-exclusion chromatography using the Superdex 200 10/300 GL column (GE Healthcare). The purity of the recombinant protein was verified by SDS–PAGE and fractions with 90–95% purity were pooled and stored at −80 °C.
Recombinant HDAC1–CoREST comprised full-length HDAC1 (UniProt: Q13547) and CoREST (amino acids 86–485). HDAC2–CoREST complex comprising HDAC2 (amino acids 2–488) (UniProt: Q92769) and CoREST (amino acids 86–485) was purified from ExpiSf9 cells (Thermo Fisher Scientific). cDNAs for human HDAC1, HDAC2 and CoREST proteins were cloned into the pFastBac donor vector and the recombinant baculoviruses were constructed using the Bac-to-Bac protocol and reagents (Thermo Fisher Scientific). The HDAC1 construct was tagged on the C terminus with a FLAG tag, the HDAC2 (amino acids 2–488) construct was tagged on the N terminus with a SUMO tag, which can be cleaved in insect cells and with 6×His on the C terminus. CoREST(86–485) was tagged with a 10× His tag followed by an MBP tag on the N terminus. To improve the solubility of CoREST, six amino acids were mutated to the corresponding residues found in MIER2 (W172K F188C F191E V197A V201N F209K). These plasmids were used to prepare separate baculoviruses according to standard protocols (Bac-to-Bac Baculovirus Expression System, Thermo Fisher Scientific). The suspension ExpiSf9 cells were grown to about 5 × 106 cells per ml before protein expression. For the HDAC1/2–CoREST complex or HDAC1/2 alone expression, the ExpiSf9 cells were either co-infected with HDAC1 or HDAC2 and CoREST baculovirus, or infected with HDAC1/2 baculovirus alone. The cells were incubated for 72 h (26 °C), collected and then frozen with liquid nitrogen for future purification. Cells were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.0 cold, 300 mM NaCl, 5 mM MgCl, 15% glycerol, 1 mM TCEP, 20 mM imidazole) supplemented with 1 mM PMSF, 10 µM leupeptin, 0.5 µM aproptinin and 1 µM pepstatin A and sonicated. Lysate was clarified by centrifugation at 100,000g for 30 min and incubated with nickel affinity resin (Thermo Fisher Scientific) or FLAG resin (anti-FLAG M2 affinity gel, Sigma-Aldrich). Resin was washed with lysis buffer, followed by elution using lysis buffer with 200 mM imidazole or 200 µg ml−1 FLAG peptide. Eluate was applied to the prepacked anion exchange column (GE Healthcare) to get rid of the contaminants and further purified by size-exclusion chromatography using a Superdex 200 10/300 GL column (GE Healthcare). The purity of the recombinant protein was verified by SDS–PAGE and fractions with 90–95% purity were pooled and stored at −80 °C.
Recombinant LSD1–CoREST complex comprised LSD1 amino acids 151–852 and CoREST amino acids 308–485. LSD1 amino acids 151–852 were cloned into a pET15b vector (gift from P. A. Cole) containing an N-terminal 6×His-tag using NEBuilder HiFi DNA Assembly Master Mix (NEB, E2621L). The LSD1 constructs were expressed in BL21-CodonPlus (DE3)-RIPL competent Escherichia coli and after plating a single colony was cultivated in 2× YT with 100 mg l−1 ampicillin at 37 °C and expression was induced at an optical density of 600 nm (OD600) of 1.0 by adding 0.3 mM isopropyl β-d-thiogalactoside (IPTG) and grown for 5 h at 25 °C. CoREST(308–485) was expressed from a pGEX vector (gift from A. Mattevi). The plasmid was transformed into BL21-CodonPlus (DE3)-RIPL E. coli cells and after plating a single colony was cultivated in LB medium with 100 mg l−1 ampicillin at 37 °C and expression was induced at OD600 of 0.8 by adding 0.25 mM IPTG and grown overnight at 17 °C. The cells were pelleted by centrifugation at 4,000g for 30 min and stored at −80 °C before purification. All of the purification steps were performed at 4 °C. Pellets of CoREST and LSD1 were resuspended in lysis buffer (50 mM NaH2PO4 pH 8.0, 300 mM NaCl, 5% glycerol, 7.5 mM imidazole supplemented with PMSF, DNase and EDTA-free Roche protease inhibitor cocktail) at a weight ratio of 1:1.5, respectively. Cells were disrupted by sonication, clarified by centrifugation and passed through nickel-affinity resin as before. The eluent was then loaded onto GST resin equilibrated in GST affinity buffer (50 mM NaH2PO4 pH 8.0, 300 mM NaCl, 5% glycerol, 1 mM DTT, 1 mM EDTA) and the GST-tag was cleaved on the resin after incubation with GST-PreScission protease (APEXBIO) overnight at 4 °C. The protein was eluted by washing the column with GST affinity buffer, concentrated and subsequently gel-filtered on a Superdex 200 10/300 GL column equilibrated in storage buffer as before. The purity of the complex was verified by SDS–PAGE and fractions with 90–95% purity were pooled and stored at −80 °C.
Recombinant LSD1–CoREST–HDAC complex comprised full-length LSD1 (UniProt: O60341) or LSD1(Δ77–86), full-length HDAC1 (UniProt: Q13547) and N-terminally truncated CoREST (amino acids 86–485) (UniProt: Q9UKL0) or N-terminal Cys CoREST17. The pcDNA3 vector was used to create plasmids encoding the different proteins. The CoREST constructs contained an N-terminal 10×His–3×FLAG tag followed by a TEV protease cleavage site. The constructs for ternary complex were co-transfected into suspension-grow HEK293F cells (Thermo Fisher Scientific) with polyethylenimine (PEI) (Sigma-Aldrich) and collected after 48 h. Cells were resuspended in lysis buffer (50 mM HEPES, pH 7.5, 100 mM KCl, 5% glycerol, 0.3% Triton X-100, 1× Roche EDTA-free cOmplete protease inhibitor cocktail) and sonicated. The lysates were clarified by centrifugation at 12,000 rpm for 30 min, and the supernatant was incubated with anti-FLAG M2 affinity gel (Sigma-Aldrich). The affinity gel was washed twice with lysis buffer and twice with SEC buffer (50 mM HEPES, pH 7.5, 50 mM KCl, 0.5 mM TCEP) followed by the incubation with TEV protease overnight at 4 °C. The complex was further purified by size-exclusion chromatography using the Superose 6 10/300 column (GE Healthcare). The purity of the complex was verified by SDS–PAGE and fractions with 90–95% purity were pooled and supplemented with 5% glycerol and stored at −80 °C.
Fluorescein labelling of LHC
The fluorescein labelling of the LSD1–CoREST–HDAC1 complex was purified as described above. A Cys point mutagenesis was conducted next to the TEV protease cleavage site of N-terminally truncated CoREST for the ligation reaction with NHS-fluorescein60. A 2 mM NHS-fluorescein was incubated with 500 mM mercaptoethanesulfonate (MESNA) in the reaction buffer (100 mM HEPES, pH 7.5, 50 mM KCl, 1 mM TCEP) for 4 h at room temperature in the dark for transesterification. The LSD1–CoREST–HDAC1 complex purified by FLAG M2 affinity gel was washed with reaction buffer and incubated with TEV protease for 5 h at 4 °C. The complex was then mixed with 500 µl of the fluorescein/MESNA solution to make a final concentration of 0.5 mM fluorescein and 125 mM MESNA. The mixture was incubated for 48 h at 4 °C in the dark. The complex was desalted by a Zeba spin desalting column (7 kDa MWCO) and further purified by size-exclusion chromatography using a Superose 6 10/300 column (GE Healthcare). Fluorescein-labelling efficiency was analysed by SDS–PAGE and fluorescence gel imaging (Amersham Typhoon FLA 9500, Cytiva). The purity of the complex was verified by SDS–PAGE and fractions with 90–95% purity were pooled and supplemented with 5% glycerol and stored at −80 °C.
FP measurements
Titration of KBTBD4
Recombinant WT KBTBD4 was diluted to 15 µM in a one-to-one mixture of ligand buffer (50 mM Tris-HCl, pH 8.0 cold, 150 mM NaCl, 1 mM TCEP, 10% glycerol) and LHC buffer (20 mM HEPES pH 7.5, 1 mM TCEP, 2 mg ml−1 BSA, 0.1% Tween-20, ±100 µM InsP6) containing 10 nM JL1 with or without 20 nM recombinant LHC, HDAC1–CoREST, HDAC2–CoREST, HDAC1, HDAC2 or LSD1–CoREST. This was aliquoted in triplicate into a black 384-well plate (Corning), followed by twofold serial dilution in assay buffer containing 10 nM JL1 with or without 20 nM recombinant LHC, HDAC1-CoREST, HDAC2-CoREST, HDAC1, HDAC2 or LSD1–CoREST (final volume, 25 µl). The plate was incubated at room temperature for 1 h and read (1,700 ms integration) using the SpectraMax i3x system with a rhodamine FP cartridge and SoftMax Pro software (Molecular Devices). Wells containing only assay buffer were used for background subtraction. The G-factor was adjusted to set the polarization of assay buffer with 10 nM JL1 and 200 nM LHC only to a reference value of 27 mP. Curves were fitted to the sigmoidal, 4PL model in GraphPad Prism 9.
Titration of SAHA or UM171
Recombinant WT KBTBD4 (5 µM) and recombinant LHC (20 nM) were diluted to in a one-to-one mixture of ligand buffer (50 mM Tris-HCl, pH 8.0 cold, 150 mM NaCl, 1 mM TCEP, 10% glycerol) and LHC buffer (20 mM HEPES pH 7.5, 1 mM TCEP, 2 mg ml−1 BSA, 0.1% Tween-20, 100 µM InsP6) containing 10 nM JL1 and 10 µM SAHA or UM171. This was aliquoted in triplicate into a black 384-well plate (Corning), followed by twofold serial dilution in assay buffer containing 10 nM JL1, recombinant WT KBTBD4 (5 µM) and recombinant LHC (20 nM) (final volume, 25 µl). The plate was incubated at room temperature for 1 h and read (1,700 ms integration) using the SpectraMax i3x system with a rhodamine FP cartridge and SoftMax Pro software (Molecular Devices). Wells containing only assay buffer were used for background subtraction. The G-factor was adjusted to set the polarization of assay buffer with 10 nM JL1, 5 µM KBTBD4 and 200 nM LHC only to a reference value of 27 mP. Curves were fit to the sigmoidal, 4PL model in GraphPad Prism 9.
Microscale thermophoresis measurements
MST assays were performed with the Monolith NT.115 (NanoTemper) system using the Nano BLUE mode. The exciting laser power was set at 50% and MST power was set to medium. KD values were calculated using MO.analysis (v.2.3) software with the quadratic equation binding KD model shown below:
$$AB=\frac{({A}_{{\rm{T}}}+{B}_{{\rm{T}}}+{K}_{{\rm{D}}})-\sqrt{{({A}_{{\rm{T}}}+{B}_{{\rm{T}}}+{K}_{{\rm{D}}})}^{2}-4({A}_{{\rm{T}}}{B}_{{\rm{T}}})}}{2}$$
Titration of KBTBD4
Fluorescein-labelled LHC (200 nM) was titrated with WT KBTBD4 in the absence or presence of DMSO or UM171 (50 µM) in the MST-binding assays at 23 °C. WT KBTBD4 (up to 11.7 μM) was prepared with a twofold serial dilution for titrating with fluorescein–LHC. Then, 50 μM UM171 or an equivalent amount of DMSO were added. LHC (or LSD1–CoREST) at a final concentration of 200 nM was then added, mixed well and incubated for 10 min for equilibration before transferring to MST premium capillaries. Prism 9 was used to fit the data to a four-parameter dose–response curve.
Histone H3K9ac synthesis
The depsipeptide as Fmoc-Thr(OtBu)-glycolic acid was synthesized based on a reported two-step protocol61. Then, H3K9ac(1–34) with a sequence as ARTKQTARKS-TGGKAPRKQL-ATKAARKSAP-A-TOG-G was synthesized by standard solid-phase peptide synthesis and purified by reversed-phase HPLC. The Fmoc-protected amino acids were purchased from Novabiochem except for Fmoc-Lys(Ac)-OH (EMD Millipore 852042). F40 sortase was expressed and purified as reported previously, and bacterial expression and purification of Xenopus laevis globular H3 (gH3; amino acids 34–135 C110A) were performed also according to a previous protocol61. Next, the F40-sortase-catalysed histone H3 ligation reaction was carried out between the H3K9ac (amino acids 1–34; note that the C-terminal residue is extruded) peptide and the gH3. The reaction mixture was purified by ion-exchange chromatography to obtain pure semisynthetic histone H3K9ac (C110A) characterized by MALDI-TOF MS as reported previously62.
Octamer refolding and nucleosome reconstitution
146 bp Widom 601 DNA was prepared according to previously reported methods used for the nucleosome reassembly63. Bacterial expression and purification of X. laevis core histones H2A, H2B and H4 were then carried out, followed by assembly of the histone octamer and refolding as previously reported64. The octamer was purified by size-exclusion chromatography using the Superdex 200 10/300 GL column (GE Healthcare) and was used for nucleosome assembly with 146 bp 601 Widom DNA as reported previously65. The final mixture was subjected to HPLC purification (Waters, 1525 binary pump, 2489 UV-Vis detector) with a TEKgel DEAE ion-exchange column to purify the final nucleosome product. The purified nucleosome containing H3K9ac was analysed by native TBE-gel with EtBr staining, as well as SDS–PAGE gel and then western blot analysis using anti-H3K9ac antibodies65.
Analysis of LHC complex deacetylation of acetylated nucleosome
The general deacetylation assay was set up as reported previously66. The LHC complex was diluted into the pH 7.5 reaction buffer containing 50 mM HEPES, 100 mM KCl, 0.2 mg ml−1 BSA and 100 μM InsP6 to a final concentration of 90 nM. After the addition of KBTBD4 to a final concentration of 300 nM and/or UM171 (in final 10% DMSO) to a final concentration of 10 μM, the solution was pre-incubated for 15 min at ambient temperature. After chilling on ice for 3 min, the deacetylation reaction was initiated with the addition of H3K9ac nucleosome to a final concentration of 100 nM, and all of the reaction solutions were incubated for 120 min at 37 °C. Different aliquots were taken at timepoints of 0, 30 min, 60 min, 90 min and 120 min. Each aliquot was quenched with an SDS-loading buffer containing 20 mM EDTA, and was heated at 95 °C for 3 min. After running SDS–PAGE and iBlot transfer to nitrocellulose membranes, western blot analysis was performed with anti-H3K9ac primary antibody (Abcam, AB32129, 1:2,000), followed by the goat anti-rabbit secondary antibody (Cell Signaling Technology, 7074S, 1:2,000). Western blot analysis with anti-H3 (Abcam, AB1791, 1:2,000) was used as the loading control. Imaging analysis with chemiluminescence on GeneSys was quantified using ImageJ software62. All intensity values were fit to a single-phase exponential decay curve with constrain Y0 = 1, plateau=0 (GraphPad Prism Ten). Each plotted point represents two replicates for the kinetic parameter V/[E] calculation.
HDAC1/2 activity assays
Recombinant HDAC1 (BPS Bioscience 50051) or HDAC2 (BPS Bioscience 50002) were diluted to 6 nM (1.2×) into buffer containing 50 mM HEPES, pH 7.5, 100 mM KCl, 0.5 mg ml−1 BSA, 0.001% Tween-20 and 25 μl added to wells of a white, 384-well microtitre plate (Corning 3572). Test compounds were added in serial dilution (1:2 titration, 15-point, cmax = 10 μM) using a D300 digital dispenser (Hewlett-Packard), and allowed to equilibrate for 1 h at room temperature. Then, 5 μl of 6× MAZ1600 HDAC substrate28 was added (final HDAC1/2 concentration 5 nM; final MAZ1600 concentration 18 μM) and deacetylase activity was allowed to proceed for 45 min at room temperature. Next, 5 μl of 7× developer solution was added (150 nM trypsin + 40 μM LBH589 final concentrations) and the plate was incubated for 30 min at room temperature. 7-Amino-4-methyl coumarin fluorescence was measured on the Tecan Spark plate reader: 350/20 nm excitation, 460/10 nm emission. The assay floor (background) was defined with the 10 μM LBH589 dose, and the assay ceiling (top) was defined through a no-inhibitor control. Data were background-corrected, normalized and Prism 9 was used to fit the data to a four-parameter dose–response curve.
TR-FRET measurements
Unless otherwise noted, experiments were performed in white, 384-well microtitre plates (Corning, 3572) at a 30 μl assay volume, or white, 384-well low-volume microtitre plates (PerkinElmer, 6008280). TR-FRET measurements were acquired on a Tecan SPARK plate reader with SPARKCONTROL software v.2.1 (Tecan) with the following settings: 340/50 nm excitation, 490/10 nm (Tb) and 520/10 nm (FITC, AF488) emission, 100 μs delay, 400 μs integration. The 490/10 nm and 520/10 nm emission channels were acquired with a 50% mirror and a dichroic 510 mirror, respectively, using independently optimized detector gain settings unless specified otherwise. The TR-FRET ratio was taken as the 520/490 nm intensity ratio on a per-well basis.
Ternary complex measurements by TR-FRET
Titration of UM171
Recombinant WT 6×His–KBTBD4 (40 nM), fluorescein-labelled LSD1–CoREST–HDAC complex (40 nM) and CoraFluor-1-labelled anti-6×His IgG (20 nM)33 were diluted into a one-to-one mixture of ligand buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM TCEP, 10% glycerol) and LHC buffer (20 mM HEPES, pH 7.5, 1 mM TCEP, 2 mg ml−1 BSA, 0.1% Tween-20, 100 μM InsP6), with or without 100 μM SAHA, and 10 μl was added to wells of a white, 384-well low-volume microtitre plate (PerkinElmer, 6008280). UM171 was added in serial dilution (1:3 titration, 10-point, cmax = 10 μM) using a D300 digital dispenser (Hewlett-Packard) and allowed to equilibrate for 1 h at room temperature before TR-FRET measurements were taken. Data were background-corrected from wells containing no UM171. Prism 9 was used to fit the data to a four-parameter dose–response curve.
Titration of UM171 and InsP6
Recombinant WT 6×His–KBTBD4 (40 nM), fluorescein-labelled LSD1-CoREST-HDAC complex (40 nM) and CoraFluor-1-labelled anti-6×His IgG (20 nM)33 were diluted into were diluted into a one-to-one mixture of ligand buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM TCEP, 10% glycerol) and LHC buffer (20 mM HEPES, pH 7.5, 1 mM TCEP, 2 mg ml−1 BSA, 0.1% Tween-20) and 10 μl was added to wells of a white, 384-well low-volume microtitre plate (PerkinElmer, 6008280). UM171 was added in serial dilution (1:10 titration, 5-point, cmax = 10 μM) and InsP6 was added in serial dilution (1:10 titration, 6-point, cmax = 100 μM) using a D300 digital dispenser (Hewlett-Packard) and allowed to equilibrate for 1 h at room temperature before TR-FRET measurements were taken. Data were background-corrected from wells containing no UM171 and no InsP6. Prism 9 was used to fit the data to a four-parameter dose–response curve.
Titration of fluorescein-labelled LSD1–CoREST–HDAC complex
Recombinant WT 6×His–KBTBD4 (10 nM, 2×) and CoraFluor-1-labelled anti-6×His IgG (5 nM, 2×)33 were diluted into LHC buffer, with or without 10 μM UM171, and 5 μl added to wells of a white, 384-well low-volume microtitre plate (PerkinElmer, 6008280). Serial dilutions of fluorescein-labelled LSD1–CoREST–HDAC complex (1:2 titration, 10-point, cmax = 1,000 nM, 2×) were prepared in ligand buffer and 5 μl was added to wells of the same plate (final volume, 10 μl; final 6×His–KBTBD4 concentration, 5 nM; final CoraFluor-1-labelled anti-6×His IgG concentration, 2.5 nM; fluorescein-labelled LSD1–CoREST–HDAC complex cmax, 500 nM). The plate was allowed to equilibrate for 1 h at room temperature before TR-FRET measurements were taken. Data were background-corrected from wells containing no 6×His–KBTBD4. Prism 9 was used to fit the data to a four-parameter dose–response curve.
In vitro ubiquitination assay
The ubiquitination assays were set up similarly as previously reported67. Reactions were performed at 37 °C in a total volume of 20 µl. The reaction mixtures contained 5 mM ATP, 100 μM WT ubiquitin, 100 nM E1 protein, 2 μM E2 protein, 0.5 μM neddylated RBX1–CUL3, 0.5 µM WT KBTBD4 (unless otherwise indicated), 10 µM UM171/DMSO with 25 mM Tris-HCl (pH 7.5), 20 mM NaCl, 10 µM InsP6 and 2.5 mM MgCl2 as reaction buffer. Substrate fluorescein–LHC at 0.5 µM was preincubated with everything except E1 in the reaction mixture at 37 °C for 5 min before adding E1 to initiate the reaction. The reactions were quenched at the indicated timepoints by adding SDS loading buffer containing reducing agent β-mercaptoethanol. The reaction samples were resolved on SDS–PAGE gels and analysed by Colloidal Blue staining and western blotting.
Base editor scan
The sgRNA libraries were designed as described previously68 to include all sgRNAs (NG protospacer-adjacent motif) targeting exonic and flanking ±30 bp into the intronic regions of canonical isoforms of KBTBD4 (ENST00000430070.7) and HDAC1 (ENST00000373548.8), excluding those with TTTT sequences as well as negative (nontargeting, intergenic) and positive (essential splice site) controls. The library was synthesized as an oligonucleotide pool (Twist Biosciences) and cloned into pRDA_478 and pRDA_479 following published workflows. Lentivirus was produced and titred by measuring cell counts after transduction and puromycin selection. Cells were transduced with library lentivirus at an MOI < 0.3 and selected with puromycin for 5 days. Cells were then expanded and split into three replicate subcultures and treated with DMSO or 1 µM UM171. After 24 h, cells were sorted on a MoFlo Astrios EQ Cell Sorter (Beckman Coulter), collecting the top 10% GFP+ and unsorted (GPF±) cells. Genomic DNA was isolated using the QIAamp DNA Blood Mini kit, and sgRNA sequences were amplified using barcoded primers, purified by gel extraction and sequenced on the Illumina MiSeq platform as previously described52,69. At all steps, sufficient coverage of the library was maintained in accordance with published recommendations.
Data analysis was performed using Python (v.3.9.12) with Biopython (v.1.78), Pandas (v.1.5.1), SciPy package (v.1.10.0) and NumPy (v.1.23.4). sgRNA enrichment was calculated as previously described52,69. In brief, sequencing reads matching each sgRNA were quantified as reads per million, increased by a pseudocount of 1, log2-transformed, normalized to the plasmid library and replicate-averaged. Sorted GFP+ abundances were normalized to unsorted abundances. The mean value for non-targeting controls was subtracted to calculate the final enrichment value for each sgRNA; this value is referred to as the normalized log2[fold change in sgRNA enrichment]. sgRNAs with zero counts in the plasmid libraries were excluded from further analysis.
sgRNAs with scores of >4 s.d. above or below the mean of intergenic negative controls were considered to be enriched or depleted, respectively. sgRNAs targeting KBTBD4 and HDAC1 were classified based on expected editing outcome, assuming any C or A within the editing window (protospacer +4 to +8) of cytidine and adenosine base editors, respectively, is converted to T or G. sgRNAs were placed in one of six mutually exclusive classes: in order of assignment priority: (1) nonsense; (2) missense; (3) silent; (4) UTR-intronic; (5) non-editing (no Cs and/or As); (6) negative controls (does not target gene). Library sgRNA annotations and base editor scanning data are provided in Supplementary Data 7–14. Scatter and line plots were generated using matplotlib (v.3.7.1).
Linear clustering analysis
Per-residue sgRNA enrichment scores were estimated as previously described45. In brief, LOESS regression was performed on using the lowess function of the statsmodels package (v.0.13.5) in Python (v.3.9.12) with a 20 amino acid sliding window (‘frac = (20 AA/L)’, where L is the total length of the protein), and ‘it = 0’ to fit observed log2[fold change in sgRNA enrichment], hereafter the sgRNA enrichment score, as a function of amino acid position. Only sgRNAs that are predicted to result in missense mutations were used. For amino acid positions that were not targeted by sgRNAs, enrichment scores were interpolated by performing quadratic spline interpolation on the LOESS output scores using the interp1d function of the SciPy package (v.1.10.0).
To assess statistical significance of the resulting clusters, we simulated a null model of random sgRNA enrichment scores. Amino acid positions of sgRNAs were kept fixed while sgRNA enrichment scores were randomly shuffled, and per-residue enrichment scores were recalculated by performing LOESS regression and interpolation on the randomized sgRNA enrichment scores for each of 10,000 permutations. Empirical P values were calculated for each amino acid by comparing its observed resistance score to the null distribution of random resistance scores. Empirical P values were adjusted using the Benjamini–Hochberg procedure to control the FDR to ≤0.05. Finally, linear clusters were called by identifying all contiguous intervals of amino acids with adjusted P ≤ 0.05. For plotting, adjusted P values were increased by a pseudocount of 10−4, log10-transformed and multiplied by −1.
Genotyping
Genomic DNA was purified using the QIAamp DNA Blood Mini (Qiagen) or QuickExtract DNA Extraction Solution (Biosearch Technologies) according to the manufacturer’s protocol. We subjected 100 ng of DNA to a first round of PCR (25–28 cycles, Q5 hot-start high-fidelity DNA polymerase (New England Biolabs)) to amplify the locus of interest and attach common overhangs. Then, 1 µl of each PCR product was amplified in a second round of PCR (8 cycles) to attach barcoded adapters. Final amplicons were purified by gel extraction (Zymo) and sequenced on an Illumina MiSeq. Data were processed using CRISPResso270 using the following parameters: –quantification_window_size 20 –quantification_window_center -10 –plot_window_size 20 –exclude_bp_from_left 0 –exclude_bp_from_right 0 –min_average_read_quality 30 –n_processes 12 –base_editor_output.
Generation of HDAC1 mutant clones
sgRNAs enriched in the base editing screens were ordered as synthetic oligonucleotides (Azenta/Genewiz), annealed, and ligated into either pRDA_478 or pRDA_479. The plasmids were transfected into HEK293T cells using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. Then, 48 h after transduction, cells were selected with 2 µg ml−1 puromycin (Thermo Fisher Scientific) for 3 days, then sorted for single-cell clones on the BD FACSAria Cell Sorter (BD Biosciences). Single-cell clones were validated by genotyping and the stability of mutants was assessed by immunoblotting. sgRNA sequences and annotations, as well as primer sequences used for genotyping are provided in Supplementary Tables 3 and 4, respectively.
Single guide validation in K562
sgRNAs enriched in the KBTBD4 CBE screen were ordered as synthetic oligonucleotides (Azenta/Genewiz), annealed and ligated into SpG Cas9 NG PAM of the pRDA_256 plasmid. Lentivirus was produced as described above and transduced into CoREST–GFP K562 cells. After puromycin selection, cells were collected and validated by genotyping. sgRNA sequences and annotations, as well as primer sequences used for genotyping are provided in Supplementary Tables 3 and 4, respectively.
Degradation assay of KBTBD4 mutants
K562 KBTBD4-null CoREST–GFP cells were generated as described above. KBTBD4 overexpression constructs were cloned into pSMAL mCherry and point mutations were introduced into coding regions using standard PCR-based site-directed mutagenesis techniques. Lentiviral particles carrying the overexpression constructs were produced and used to transduce K562 KBTBD4-null CoREST–GFP cells as described above. Then, 48 h after transduction, cells were treated with 1 µM UM171, or 0.1% DMSO for 24 h. The GFP+ percentage was measured for mCherry+ cells in each condition and FACS gating schemes are shown in Supplementary Fig. 1c.
Production of eVLPs
Engineered virus-like particles (eVLPs) were produced as previously described71. In brief, Gesicle Producer 293T cells were seeded into T-75 flasks (Corning) at a density of 5 × 106 cells per flask. After 20–24 h, a mixture of plasmids expressing VSV-G (400 ng), MMLVgag–pro–pol (3,375 ng), MMLVgag–3×NES–ABE8e (1,125 ng) and an sgRNA (4,400 ng) were co-transfected into each T-75 flask using jetPRIME transfection reagent (Polyplus) according to the manufacturer’s protocols. Then, 40–48 h after transfection, the producer cell supernatant was collected and centrifuged for 10 min at 4 °C and 2,000g to remove the cell debris. The clarified eVLP-containing supernatant was filtered through a 0.45 μm PVDF filter (Sigma-Aldrich). The filtered supernatant was concentrated by ultracentrifugation using a cushion of 20% (w/v) sucrose (Sigma-Aldrich) in PBS. Ultracentrifugation was performed at 26,000 rpm for 2 h at 4 °C using an SW28 rotor in an Optima XE-90 Ultracentrifuge (Beckman Coulter). After ultracentrifugation, eVLP pellets were resuspended in cold PBS (pH 7.4). eVLPs were frozen and stored at −80 °C. eVLPs were thawed on ice immediately before use and repeated freeze–thaw was avoided.
eVLP transduction in cell culture
K562 cells were plated for transduction in 96-well plates (Cellstar Greiner Bio-one) at a density of 50,000 cells per well with 5 µg ml−1 polybrene (Santa Cruz Biotechnology) medium. BE-eVLPs were added directly to the culture medium in each well. Then, 50 µl of fresh medium was added after 6 h, and another 100 µl of medium was added at 48 h after transduction. Then, 72 h after transduction, cellular genomic DNA was isolated and genotyped as described below. Transduced cells were allowed to recover for 7–10 days before degradation assays were performed.
Cryo-EM sample preparation and data collection
To assemble the complex of KBTBD4–UM171–LHC for the cryo-EM study, the individually isolated KBTBD4 protein and co-expressed LHC complex were mixed in stoichiometric amounts with 1 μM UM171 added and subsequently applied to the Superose6 increase gel-filtration column (Cytiva) in a buffer containing 40 mM HEPES, pH 7.5, 50 mM KCl, 100 µM InsP6 and 0.5 mM TCEP (Tris(2-carboxyethyl)phosphine). The isolated complex was then cross-linked with 37.5 mM glutaraldehyde at room temperature for 6 min and quenched with 1 M Tris-HCl pH 8.0. The cross-linked sample was snap-frozen for future use.
To prepare grids for cryo-EM data collection, a QuantiFoil Au R0.6/1 grid (Electron Microscopy Sciences) was glow discharged for 30 s at 20 mA with a glow discharge cleaning system (PELCO easiGlow). 3.0 μl of the purified KBTBD4-UM171-LHC complex at 0.7 mg ml−1 was applied to a freshly glow-discharged grid. After incubating in the chamber at 10 °C and 100% relative humidity, the grids were blotted for 3 s with a blotting force of zero, then immediately plunge-frozen in liquid ethane using the Vitrobot Mark IV system (Thermo Fisher Scientific). Data collection was performed on the FEI Titan Glacios transmission electron microscope (Thermo Fisher Scientific) operated at 200 kV at the Arnold and Mabel Beckman Cryo-EM Center of the University of Washington. The automation scheme was implemented using the SerialEM72 software (v.4.1.8) using beam-image shift73 at a nominal magnification of ×105,000, resulting a physical pixel size of 0.885 Å. The images were acquired on a K3 camera direct detector. The dose rate was set to 10 e− Å−2 s−1, and the total dose of 50 electrons per Å2 for each image was fractionated into 99 electron-event representation frames. Data were collected in four sessions with a defocus range of 0.8–1.8 μm. In total, 6,839 videos were collected.
To prepare grids for the cryo-EM study of apo KBTBD4, a QuantiFoil Au R1.2/1.3 grid (Electron Microscopy Sciences) was glow discharged for 30 s at 20 mA with a glow discharge cleaning system (PELCO easiGlow). Then, 3.0 μl of purified KBTBD4, with a final concentration of 0.1% n-decyl-β-d-maltoside and a protein concentration of 4 mg ml−1 was applied to a freshly glow-discharged grid. After incubating in the chamber at 10 °C and 100% relative humidity for 60 s, grids were blotted for 3 s with a blotting force of zero, then immediately plunge-frozen in liquid ethane using a Vitrobot Mark IV system (Thermo Fisher Scientific). Data collection was performed on the FEI Titan Glacios transmission electron microscope (Thermo Fisher Scientific) operated at 200 kV at the Arnold and Mabel Beckman Cryo-EM Center of the University of Washington. Automation scheme was implemented using the SerialEM72 software using beam-image shift73 at a nominal magnification of ×105,000, resulting a physical pixel size of 0.885 Å. The images were acquired on a K3 camera direct detector. The dose rate was set to 10 e− Å−2 s−1, and the total dose of 50 e− Å−2 for each image were fractionated into 99 electron-event representation frames. Data were collected in four sessions with a defocus range of 0.8–1.8 μm. In total, 11,263 videos were collected.
Image processing and 3D reconstruction
For the KBTBD4–UM171–LHC structure, a total of 10,816 videos were collected and imported into CryoSPARC74 followed by patch motion correction and patch contrast transfer function (CTF) estimation. In total, 10,637 micrographs were retained after filtering the micrographs with CTF parameters and manual inspection. The blob picker job in CryoSPARC was able to pick 7,133,729 particles, which were further extracted and subjected to 2D classification. After five rounds of cleaning by 2D classification, 928,437 particles were selected and subjected to ab initio reconstruction. Subsequently, all of the particles were used for heterogenous refinement. After one extra round of cleaning up by heterogenous refinement, 186,315 particles from good reconstruction were selected to get re-extracted without Fourier cropping. The homogenous refinement and non-uniform refinement75 helped to reach an overall resolution of 3.93 Å. To optimize the map for the KELCH-repeat domain, two different soft masks focused on the BTB-BACK-KELCH domain in chain A and HDAC1–CoREST-KELCH in chain B was applied to local refinement, respectively, which led to a further improved resolution of 3.77 Å and 3.86 Å. The two maps provided clearer density for the KBTBD4 protomer A and CoREST. Further details about the data processing are provided in Extended Data Fig. 4.
For the apo KBTBD4 structure, in total, 11,263 videos were collected and imported into CryoSPARC74 followed by patch motion correction and patch CTF estimation. In total, 10,057 micrographs were retained after filtering the micrographs with CTF parameters and manual inspection. The blob picker job in CryoSPARC was able to pick 1,039,200 particles, which were further extracted and subjected to 2D classification. A total of 147,826 particles was used for primary ab initio reconstruction, from which the templates were generated, and template picker was conducted to pick 8,280,266 particles. After two rounds of cleaning by 2D classification, 340,735 particles were selected and subjected to Topaz picking. Subsequently, after two rounds of cleaning by 2D classification, 766,539 particles were used for ab initio reconstruction and heterogenous refinement. After one extra round of cleaning up by heterogenous refinement, 572,349 particles from good reconstruction were selected to get re-extracted without Fourier cropping. The homogenous refinement and non-uniform refinement75 helped to reach an overall resolution of 3.83 Å. Further details about the data processing are provided in Extended Data Fig. 5.
Model building and refinement
The initial structural models of the KBTBD4 dimer and the HDAC1–CoREST–ELM–SANT1 complex was predicted with AlphaFold-Multimer in Google ColabFold276. The structural models of KBTBD4 BTB-BACK domain, KELCH-repeat domain, and HDAC1–CoREST were separately fit into the cryo-EM map using UCSF ChimeraX-1.7 (rc2023.12.12)77. The resulting model was subsequently rebuilt in Coot (v.0.9.8.91)78 based on the protein sequences and the EM density and was further improved by real-space refinement in PHENIX (v.1.20.1-4487-000)79,80. The structure figures were made using PyMOL (v.2.5.4)81.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.