Metagenomic and bioinformatic approaches
Soil sample collection
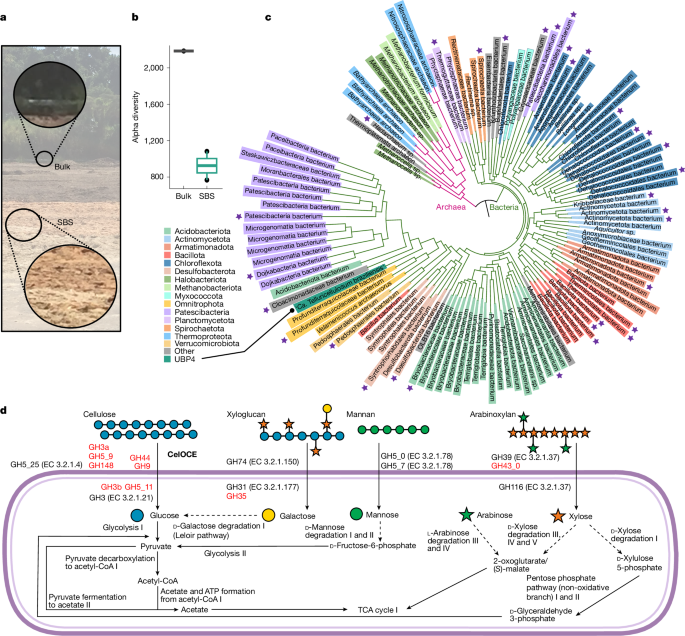
Soil samples were collected from a sugarcane mill in Quatá, São Paulo, Brazil, where residual sugarcane bagasse had been stored over 20 years (Fig. 1a). After mechanically removing the surface bagasse layer, samples were collected at the soil surface and from a depth of 20 cm. These samples are referred to as sugarcane bagasse-covered soil (SBS). A control sample was collected from nearby soil without bagasse coverage. All samples were immediately frozen in liquid nitrogen and stored at −80 °C until further processing.
Nucleic acid extraction
Microbial DNA was extracted from both SBS and bulk control soils using the FastDNA Spin Kit for Soil (MP Biomedicals). Ten grams of soil samples were pulverized with an oscillating ball mill (TE-350, Tecnal) and used to conduct five extraction batches of 2 g each, resulting in five separate DNA extracts. These extracts were then transferred to Lysing Matrix E Tubes, external contaminants were solubilized with MT buffer and sodium phosphate buffer was added for cell lysis. The samples were homogenized using a FastPrep FP120 instrument (MP Biomedicals). Protein precipitation solution was added to the supernatant to separate nucleic acids from cellular debris, followed by centrifugation at 14,000g for 5 min. The resulting supernatant was then mixed with a binding matrix, incubated for 3 min and transferred to a spin filter. After centrifugation (14,000g for 1 min), the pellet was washed with a salt–ethanol solution (SEWS-M), dried and resuspended in ultrapure water. Further purification was performed using the PowerClean DNA Clean-Up Kit (Mo Bio Laboratories). DNA quality was assessed by 0.8% (w/v) agarose gel electrophoresis.
16S rRNA amplicon sequencing and analysis
The V4 region of the 16S rRNA gene was amplified in triplicate using the primers 515F and 806R. Paired-end sequencing (2 × 300 bp) was performed on an Illumina MiSeq platform (V3 kit, 600 cycles) using the MiSeq reporter software at the high-performance sequencing facility of the Brazilian Biorenewables National Laboratory (LNBR). The ZymoBIOMICS microbial community DNA standard II served as a positive control. For taxonomic analysis, paired-end reads were quality-checked with FastQC v.0.12.0 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and filtered with Trimmomatic44 v.0.36 to remove adapters and low-quality reads. Filtered reads were merged using the fastq_mergepairs function from the Usearch v.10 package45 (minimum overlap of 50 bp and maximum error of 0.5), followed by the removal of primer and singleton sequences. The UPARSE unoise3 function was used for denoising and zOTU (zero radius OTUs) recovery. Taxonomic assignment was performed with the sintax function (cut-off 0.8, RDP database v.16)46. Further analyses were performed using the phyloseq v.1.20 package in R Studio v.1.3.1093 (https://bioconductor.org/packages/release/bioc/html/phyloseq.html). Details on read counts and diversity indices are described in Supplementary Table 15.
Metagenomic short- and long-read sequencing
Metagenomic libraries were constructed using the Nextera library preparation kit (Illumina). Quantification and quality control of the libraries were performed using quantitative PCR and the KAPA library quantification kit (Roche) and the Agilent Bioanalyzer 2100 system (Agilent Technologies). Sequencing was performed on an Illumina HiSeq 2500 device (2 × 250 bp) using the HiSeq 2500 control software. Furthermore, long-read sequencing was conducted on a MinION device (Oxford Nanopore) using the MiniKNOW v.19.12.5 software. For long-read sequencing, 1 µg of high-molecular-mass DNA from the SBS sample was prepared with SQK-LSK109 and Native Barcoding Kits.
Metagenomic de novo assembly and binning
Raw metagenomic sequences underwent quality control and trimming using FastQC v.0.12.0 and Trimmomatic v.0.36, followed by taxonomic classification with Kaiju47 v.1.7.4. Quality-filtered reads were de novo assembled using IDBA_UD v.1.1.1 with pre-correction and k-mer sizes48 of 20–60 (Supplementary Table 16). The resulting assemblies were binned using MetaWRAP v.1.349, generating initial bin sets with MetaBAT2, MaxBin2 and CONCOCT, followed by refinement and reassembly (minimum completion 55%, maximum contamination 15%). Bins were then taxonomically classified and functionally annotated using the modules Classify and Annotate_bins, respectively. Furthermore, long reads from Oxford Nanopore Technologies sequencing were used for scaffolding with SSPACE-long-reads v.1.1 with parameters: -k 5, -a 0.7, -x 1, -m 50, -o 20 and -n 1000. Final MAGs were assessed for completeness and contamination using CheckM250 v.1.0.2 and further classified with GTDB-tk against the GTDB database51 release 214. Detailed information on recovered MAGs is summarized in Supplementary Table 2. Gene prediction and annotation were performed with Prokka52 v.1.11. CAZyme and PUL annotations followed CAZy pipelines based on hidden Markov model profiles and sequence similarity26. To estimate CAZyme gene abundance, metagenomic reads were mapped to MAG gene sets using Kallisto v.0.46.1 with quant function53 and normalized abundance was expressed as transcripts per million (TPM).
Phylogenetic analysis and metabolic reconstruction
The phylogenetic profile of recovered MAGs was reconstructed using UBCG54 v.3.0, involving marker gene identification, multiple sequence alignment refinement and concatenation, and phylogeny reconstruction using Mafft55 v.7.487 and RAxML56 v.8.2.12. The resulting tree was visualized using the iTOL57 web tool v.6.9.1. Metabolic pathways in the recovered MAGs were reconstructed using gapseq58 v.1.1 and KEGG Orthology annotations. Enzyme commission (EC) numbers were assigned using KOFAMscan59 v.1.3.0 (e < 1 × 10−5). Proteins annotated as CAZymes but lacking an EC annotation had their EC transferred from characterized CAZymes of the same family recovered from the CAZy database using DIAMOND60 v.2.0.14.152. Pathway abundance was estimated by aligning metagenomic reads to binned sequences with bowtie2 (ref. 61) v.2.4.5 and calculating bin TPMs (SAMtools62 v.1.15.1). Each pathway predicted in a bin was assigned to its TPM, with total pathway abundance being the sum across relevant bins.
Pipeline for enzyme discovery from microbial dark matter
In silico protein selection approach
Protein sequences from the lignocellulolytic MAG ‘Ca. Telluricellulosum braziliensis’ belonging to the recently discovered and uncharacterized bacterial phylum UBP4 (GTDB database) were initially retrieved. Sequences lacking CAZy annotation were further analysed using HHpred63 v.3.3 and those exhibiting remote homology (10–25% sequence similarity) to proteins involved in carbohydrate breakdown and metabolism were selected for heterologous expression and biochemical assays (Supplementary Table 3).
Gene synthesis, heterologous expression and purification
The eight selected sequences were codon optimized for E. coli expression and synthesized with an N-terminal 6×His-tag. Next, E. coli BL21(DE3) cells were transformed with the target genes in pET-28a(+) expression vectors. Transformants were grown in Luria–Bertani (LB) medium (0.5% (w/v) yeast extract, 1% (w/v) tryptone and 1% (w/v) sodium chloride) at 37 °C to an optical density at 600 nm (OD600 nm) of approximately 0.8. Protein expression was induced with 0.4 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) (Sigma Aldrich) at 18 °C for 16 h. Cells were collected by centrifugation (13,000g, 15 min, 4 °C) and resuspended in lysis buffer (20 mM sodium phosphate, pH 7.4, 300 mM NaCl, 5 mM imidazole, 1 mM phenylmethylsulfonyl fluoride (PMSF), 25 U ml–1 Turbo nuclease, 0.1 mg ml–1 lysozyme, 1.2 mg ml–1 deoxycholic acid). The lysed sample was then centrifuged at 21,000g for 40 min (4 °C). Soluble protein lysates were loaded onto a 5-ml HiTrap Chelating HP column (GE Healthcare) and the 6×His-tag target proteins were eluted using an imidazole gradient (up to 0.5 M). Further purification was achieved through size-exclusion chromatography on a HiLoad 16/600 Superdex 75/200 pg column (Cytiva) equilibrated with 20 mM sodium phosphate (pH 7.4) and 150 mM NaCl. Protein purity was assessed by SDS–PAGE and protein concentration were determined by measuring absorbance at 280 nm using the calculated extinction coefficient (ε280 nm) for each sequence.
Activity screening assays
Recombinant proteins (Supplementary Table 3) were screened for activity against a broad panel of substrates, including polysaccharides, oligosaccharides and synthetic p-nitrophenol derivatives (Supplementary Table 7). Assays were conducted by incubating the purified protein with the substrate (0.5% (w/v) for polysaccharides or 1 mM for oligosaccharides and synthetic substrates) in 50 mM sodium acetate buffer (pH 5.0) at 40 °C for up to 24 h. Enzymatic activity on polysaccharides was assessed by quantifying reducing sugar release using the 3,5-dinitrosalicylic acid (DNS) method64. For synthetic substrates, activity was determined by monitoring the release of p-nitrophenol at 405 nm. Enzyme assays consist of at least three independent experiments.
Saccharification boosting screening
To assess the potential of purified proteins to enhance the saccharification efficiency of the T. reesei Br_TrR03 cellulase cocktail27,28, we conducted saccharification experiments using steam-exploded sugarcane bagasse as described in the ‘Lignocellulosic biomass pretreatment’ section. In the screening phase, reactions were performed with 5% (w/v) total solids and an enzyme cocktail dosage of 1 mg g–1 of pretreated bagasse. Reactions were incubated for 24 h at 40 °C in 50 mM sodium acetate buffer (pH 5.0) using a combi-D24 hybridization incubator (FinePCR). The boosting effect was assessed by determining the total reducing sugar released by the DNS method64. Saccharification assays consist of at least three independent experiments.
Trichoderma enzyme cocktail production for screening assays
The T. reesei Br_TrR03 strain was cultivated in a BioFlo/CelliGen 115 system (Eppendorf) to produce the enzyme cocktail used in the saccharification boosting screening28. The cultivation medium contained 20 g l–1 (NH4)2SO4, 1.0 ml l–1 J647 antifoaming agent (Struktol), 20 g l–1 whole yeast cells (dry mass) and 50 g l–1 total reducing sugars (TRS) from sugarcane molasses. Bioreactors were initialized with 1.0 l of medium, including a 10% (v/v) inoculum prepared from fungal spores. The cultivation process was controlled as follows: pH was maintained at 4.5 ± 0.5 using 2 M phosphoric acid and 10% (w/v) ammonium hydroxide; temperature was kept at 28.0 °C; aeration was provided at 0.7 standard litres per minute (slpm) compressed air; and an agitation cascade (400–1,000 rpm) was used to ensure that the dissolved oxygen remained above 20%. From 25 h onwards, a sugarcane molasses solution (approximately 350 g kg–1 TRS) with 1.0 ml l–1 antifoaming agent was fed at 1.3 g TRS kg–1 h–1 (based on instant bioreactor mass) until 1 h before collection, creating a non-linear feeding profile. Samples were collected every 24 h, centrifuged and the supernatants stored at −20 °C. The final fermentation broth was used for saccharification experiments, protein quantification (Lowry method, BSA standard)65 and enzymatic activity analysis.
Lignocellulosic biomass pretreatment
Sugarcane bagasse and eucalyptus residues were pretreated at the LNBR pilot plant using a SuPR 2G reactor (AdvanceBio Systems). Sugarcane bagasse underwent steam explosion using 0.5% (v/v) sulfuric acid at 140 °C for 15 min, followed by centrifugation (1,610g, 20 min) to separate the C5 stream. Eucalyptus residue was first subjected to alkaline deacetylation with 0.4% (w/w) NaOH at 70 °C for 60 min, then steam exploded with 0.25% (v/v) sulfuric acid at 190 °C for 3 min and the C5 stream was separated. The resulting pretreated sugarcane bagasse contained 53.4% cellulose, 33.3% lignin and 5.8% hemicellulose, whereas the pretreated eucalyptus residue consisted of 61.5% cellulose, 33.8% lignin and 3.3% hemicellulose.
Production and purification of CelOCE, variants and other enzymes
Site-directed mutagenesis
CelOCE variants (CelOCE(Δ2–5) and CelOCE(F33A)) were generated using inverse PCR. Primers were designed with complementary sequences longer than 15 nucleotides and a Tm of 50 °C (see Supplementary Table 17 for primer sequences). PCR amplicons were circularized using Gibson Assembly66. The resulting plasmids were transformed into E. coli BL21(DE3) cells, and variant proteins were expressed and purified following the same protocol as for the wild-type enzyme. All mutations were confirmed by Sanger sequencing.
Preparative expression and purification of CelOCE and variants
CelOCE and its variants were overexpressed in E. coli BL21(DE3) using the pET-28a(+) expression vector without a 6×His-tag. Following the same expression protocol as described in the ‘Gene synthesis, heterologous expression and purification’ section, soluble protein lysates were subjected to ion exchange chromatography on a 5-ml HiTrap Q-FF column (Cytiva). CelOCE was eluted with a saline gradient up to 0.5 M, followed by size-exclusion chromatography using a HiLoad 16/600 Superdex 75 pg column (Cytiva) equilibrated with 20 mM sodium phosphate buffer (pH 7.4), containing 150 mM NaCl. To remove excess salt and prevent precipitation, proteins were buffer-exchanged using a HiTrap Desalting 5 ml column (Cytiva). CelOCE was then doped with sub-equimolar CuSO4 to enhance stability, followed by removal of excess copper using a VIVASPIN TURBO concentrator (10 kDa molecular weight cut-off (MWCO), Sartorius). To rule out interference by contaminations, three independent enzyme preparations were included, each starting from fresh transformations and including previously unused purification columns (Supplementary Fig. 28). In all three preparations, the activity on Avicel PH-101 (Sigma Aldrich) was validated by detecting cellobionic acid (Supplementary Fig. 29).
Size-exclusion chromatography with multi-angle light scattering
Size-exclusion chromatography with multi-angle light scattering (SEC–MALS) experiments were performed to determine the molecular mass and oligomeric state of CelOCE and its variants. In brief, 100 µl of purified protein samples were injected into a Superdex 200 (10/300) analytical size-exclusion column (Cytiva) connected to a high-performance liquid chromatography (HPLC) 1260 Infinity II system (Agilent). The column was equilibrated with 20 mM HEPES buffer (pH 7.4) containing 150 mM NaCl. Elution was monitored using a DAWN8 eight-angle static light scattering detector and an Optilab refractive index monitor (Wyatt Technology). Data acquisition and molecular mass calculations for CelOCE and its variants were performed using ASTRA v.8.1.2 software (Wyatt Technology).
BacB production and purification
The Bacillus subtilis bacilysin biosynthesis protein (BacB, PDB: 3H7J) sequence was codon optimized for E. coli expression, synthesized with an N-terminal 6×His-tag and subcloned into the pET-28a(+) vector. Next, E. coli BL21(DE3) cells containing the plasmid were grown in LB medium at 37 °C to an OD600 of approximately 0.8, then induced with 0.4 mM IPTG at 18 °C for 16 h. Cells were collected by centrifugation (13,000g, 15 min, 4 °C), lysed and centrifuged as described in the ‘Gene synthesis, heterologous expression and purification’ section. Recombinant BacB was purified using nickel-affinity and size-exclusion chromatography, using the same protocols and conditions outlined in the ‘Gene synthesis, heterologous expression and purification’ section.
KdgF production and purification
The Yersinia enterocolitica subsp. enterocolitica 8081 uronate metabolism protein (KdgF, PDB: 5FPX) sequence was codon optimized for E. coli expression and synthesized with an N-terminal 6×His-tag. The gene was subcloned into the pET-28a(+) expression vector. Expression and purification of KdgF followed the same protocol outlined in the ‘Gene synthesis, heterologous expression and purification’ section.
Cel5A production and purification
The B. subtilis endo-β-1,4-glucanase (Cel5A, GH5_2) was produced as described previously39. In brief, Cel5A was expressed in BL21(DE3)slyD– cells in LB medium at 37 °C for 4 h after induction with 0.5 mM IPTG. Collected cells were resuspended in lysis buffer (50 mM sodium phosphate, pH 7.4, 100 mM NaCl, 1 mM PMSF, 5 mM benzamidine), then lysed with lysozyme (80 μg ml–1, 30 min, on ice) and sonication. The lysate was centrifuged (10,000g, 30 min) and the supernatant was loaded onto a 5 ml HiTrap Chelating column (GE Healthcare) at 1 ml min–1. Proteins were eluted with a 0–500 mM imidazole gradient. Further purification was achieved using a 5 ml HiTrap SP HP column (Cytiva) with a 0–1 M NaCl gradient at 1 ml min–1. Size-exclusion chromatography was performed on a Superdex 75 16/60 column (Cytiva) equilibrated with 50 mM sodium phosphate, pH 7.4, 150 mM NaCl.
Cel45A production and purification
The Thermothielavioides terrestris endo-β-1,4-glucanase (Cel45A, GH45_1)40 was synthesized with an N-terminal 6×His-tag and subcloned into the pET-28a(+) vector. E. coli BL21(DE3) SHuffle cells containing the plasmid were grown in LB medium at 37 °C to an OD600 of 0.8. Cel45A expression was then induced with 0.4 mM IPTG at 18 °C and 180 rpm for 16 h. Collected cells were lysed by resuspension in lysis buffer containing sodium deoxycholate (60 mg l–1 of culture), lysozyme (20 mg l–1 of culture), 1 mM PMSF and DNase (20 µg ml–1) in buffer (20 mM sodium phosphate, pH 7.4, 300 mM NaCl, 5 mM imidazole). After incubation on ice for 1 h with gentle agitation, the lysate was centrifuged (21,000g, 45 min). The supernatant was subjected to nickel-affinity chromatography on a 5 ml HiTrap Chelating column (Cytiva), eluting the 6×His-tag protein with a 0–500 mM imidazole gradient. Final purification was achieved by size-exclusion chromatography on a HiLoad 16/600 Superdex 200 column (Cytiva) equilibrated with 20 mM sodium phosphate (pH 7.4) and 150 mM NaCl.
Cel7A production and purification
The cellobiohydrolase from T. reesei (Cel7A, GH7) was purified from the fungus secretome as described previously41. The T. reesei Br_TrR03 secretome was obtained as outlined in the ‘Trichoderma enzyme cocktail production for screening assays’ section. The secretome solution was vacuum-filtered through Miracloth (EMD Biosciences) using a 0.45-μm PES membrane and concentrated by tangential ultrafiltration (10 kDa MWCO). Buffer exchange was performed with 20 mM Bis-Tris (pH 6.5) to remove low-molecular-mass contaminants, followed by another filtration step. The filtrate was adjusted to 1.5 M (NH4)2SO4 and loaded onto a 26/10 Phenyl Sepharose Fast Flow column. Unbound material was washed off with 80% of 20 mM Bis-Tris pH 6.5 containing 2 M (NH4)2SO4, followed by elution with a descending gradient (80% to 0%) over eight column volumes. Active fractions were identified using a pNP-lactose activity assay (2 mM pNPL, 50 mM acetate pH 5.0, 30 min, 45 °C). These fractions were pooled, concentrated and desalted into 20 mM Bis-Tris (pH 6.5) using Superdex 25 HiPrep columns. The desalted protein was then loaded onto a Source 15Q 10/100 anion-exchange column and eluted with a 0–50% gradient of 20 mM Bis-Tris pH 6.5 containing 1 M NaCl over 30 column volumes. Active fractions were identified by pNP-lactose activity. The final purification step involved size-exclusion chromatography on a Superdex 75 16/60 column using 20 mM acetate buffer (pH 5.0) with 100 mM NaCl.
LPMO production and purification
The LPMOs TtAA9J from Thermothelomyces thermophilus, LsAA9A from L. similis, PaAA9E from Podospora anserina and NcAA9C from Neurospora crassa were expressed in Komagataella phaffii X-33 using the pPICZα vector and their native signal peptides. Cells were cultured in YPD medium (1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) glucose) at 30 °C and 200 rpm until glucose depletion. Protein expression was induced by adding 1% (v/v) methanol every 24 h for 72 h. The supernatants, obtained after centrifugation (13,000g, 15 min), were concentrated and buffer-exchanged into 20 mM Tris-HCl (pH 7.0) using a 10 kDa MWCO hollow fibre cartridge coupled to a tangential flow filtration system (GE Healthcare). The concentrates were applied to a DEAE-Sepharose XK 16/100 column (Cytiva) and eluted with a 0–1 M NaCl gradient, except for NcAA9C, which was applied to a CM-Sepharose XK 16/100 column (Cytiva) and eluted with a 0–1 M NaCl gradient. LPMO-containing fractions were pooled, concentrated and incubated with CuSO4 (3:1 molar ratio) on ice for 1 h. After centrifugation (20,000g, 10 min), the samples were further purified on a HiLoad 16/600 Superdex 75 pg column (Cytiva) equilibrated with 20 mM Tris-HCl (pH 7.0) and 150 mM NaCl. The final LPMO fractions were pooled, concentrated and stored at 4 °C.
Sample purity and quantification
Protein purity was assessed by SDS–PAGE (10%, w/v), followed by staining with Imperial Protein Stain (Thermo Fisher Scientific). Molecular mass under denaturing conditions was estimated using a PageRuler Prestained Protein Ladder (Thermo Fisher Scientific). Protein concentrations were determined either by the Bradford assay (Bio-Rad) or by measuring absorbance at 280 nm and using the calculated extinction coefficient (ε280) for each protein sequence.
Cellulose interaction assays
Qualitative cellulose binding assay
The cellulose binding capacity of CelOCE was evaluated qualitatively as described previously67. In brief, 80 µg of CelOCE was incubated with 5% (w/v) Avicel in 50 mM sodium acetate buffer (pH 5.2) for 24 h at 4 °C with gentle agitation. The final reaction volume was 200 µl, and incubations were performed in the presence or absence of 1 mM ASC. After incubation, insoluble cellulose was pelleted by centrifugation at 13,000g for 2 min. The supernatant, containing unbound proteins, was carefully removed. The Avicel pellet was washed twice by resuspension in buffer and centrifugation. The washed pellet was then resuspended in 200 µl of SDS-loading buffer (without dye) and heated at 95 °C for 10 min. Both the soluble (supernatant) and insoluble (pellet) fractions were analysed by SDS–PAGE using a 4–20% gradient gel.
Quantitative cellulose binding assay
The binding of CelOCE, with or without reductant (ASC), to microcrystalline (Avicel) and amorphous (PASC) cellulose was quantified as described previously29. In brief, reactions containing 5% (w/v) Avicel or 0.2% (w/v) PASC in 50 mM sodium acetate buffer (pH 5.0) were incubated with 0.1–1.5 mg ml–1 CelOCE in a final volume of 500 µl. BSA served as a negative control in the same concentration range. Reactions were incubated for 16 h at 4 °C with constant agitation using a Revolver rotator (Labnet International). Insoluble substrate with bound CelOCE was removed by centrifugation at 20,000g for 3 min. The concentration of free enzyme (biological unit) in the supernatant was determined spectrophotometrically at 280 nm. For reactions containing 1 mM ASC, 240 mM phosphoric acid was also added, and absorbance measurements were taken at 290 nm (ref. 29). Standard curves for CelOCE and BSA in the presence of 1 mM ASC were obtained at 290 nm. The experimental data were fitted using the Langmuir–Freundlich model68. Data are the mean ± s.d. of three independent experiments.
Competitive binding assay
For the BSA blocking assay, reactions containing 5% (w/v) Avicel in 50 mM sodium acetate buffer (pH 5.0) were incubated with 0.2 mg ml–1 CelOCE and/or 0.2 mg ml–1 BSA. CelOCE was tested in the absence or presence of reductant (1 mM ASC) forms. The final reaction volume was 500 µl. Incubations were performed at 4 °C with constant agitation using a Revolver rotator (Labnet International) for 2, 8, 16 and 24 h. The insoluble substrate with bound proteins was removed by centrifugation at 20,000g for 3 min. The concentration of free enzymes in the supernatant was measured using the Bradford method69. Data are the mean ± s.d. from three independent experiments.
XPS and FTIR spectroscopy
To further investigate the interaction between CelOCE and microcrystalline cellulose or bleached cellulose fibres (extracted from sugarcane bagasse), cellulose dispersions (0.4% w/v) were prepared in 20 mM sodium phosphate buffer (pH 5.4) containing 150 mM NaCl and soaked overnight. Enzyme and reductant (ASC) were then added to achieve final concentrations of 1 mM and 480 mg g–1 cellulose, respectively. The reaction mixtures were incubated at 40 °C for 72 h with orbital shaking. Two distinct washing procedures were evaluated. In the first, cellulose pellets were collected by centrifugation at 13,000g for 15 min and then subjected to multiple cycles of washing and centrifugation with deionized water until the conductivity of the wash solution approached that of pure water. The second procedure involved washing the cellulose pellets (200 mg) ten times with 30–40 ml of a 1 mM aqueous SDS solution at 40 °C, followed by rinsing with water to remove residual SDS. The surface elemental composition of the cellulose samples was acquired using a K-Alpha instrument (Thermo Fisher Scientific) with energy resolution of approximately 1 eV and the Avantage v.5.9931 software. Chemical characterization of the samples was performed using a FT-IR Spectrometer (PerkinElmer) and the Spectrum v.10 control software. Spectra were collected at a resolution of 4 cm−1, in the range of 4,000 to 700 cm−1, with a total of 128 scans. Data were analysed using the OriginPro v.2023b software (OriginLab).
Enzyme and complementary assays
Analysis of oxidized and non-oxidized oligosaccharides
Monosaccharides, cellooligosaccharides (degree of polymerization (DP) from 2 to 6), polysaccharides and their corresponding aldonic acid forms resulting from the cleavage of Avicel, PASC, sugarcane bagasse, eucalyptus, chitin, mannan, xylan, xyloglucan, arabinoxylan, β-glucan, laminarin, lichenan, starch and pectin were analysed by high-performance HPAEC–PAD using a Dionex ICS6000 system (Thermo Fisher Scientific) and the Chromeleon v.7.3 software. Reactions containing 0.1% (w/v) of the distinct polysaccharides, 50 mM sodium acetate (pH 5.0), 1 µM of the tested enzyme (CelOCE, KdgF or BacB) and 1 mM reductant were incubated for 16 h at 37 °C. All samples were homogenized by vortexing, filtered through a Millex 0.22-µm syringe filter. Then, 5 µl of each reaction were analysed on an HPAEC–PAD system equipped with a 2 × 50 mm CarboPac PA1 guard column and a 2 × 250 mm CarboPac PA1 analytical column (Thermo Fisher Scientific) maintained at 30 °C. The analysis was performed at a flow rate of 0.1 ml min–1. The column was equilibrated with 0.1 M NaOH (eluent A) and bound oligosaccharides were eluted using a gradient of 1 M sodium acetate (eluent B) as follows: 0–10% B (linear) over 10 min, 10–30% B (linear) over 25 min, 30–100% B (exponential) over 5 min, 100–0% B (linear) over 1 min and 0% B for 9 min for re-equilibration. Electrochemical detection was carried out using a gold working electrode and an Ag/AgCl pH reference electrode. Soluble cellooligosaccharides (DP 2–6, Megazyme) were used as standards. Corresponding C1-oxidized standards (DP 2–6) were produced by treating non-oxidized cellooligosaccharides with the cellobiose dehydrogenase from T. thermophilus (TtCDH, XM003664495)70. To enable quantification of cellobionic acid from all reactions, a calibration curve was generated using varying concentrations of cellobionic acid standard. Each assay consisted of at least three independent experiments. Data were analysed using the OriginPro v.2023b software (OriginLab).
Cellobionic acid detection by liquid chromatography coupled to mass spectrometry
Cellobionic acid generated by CelOCE activity on Avicel in the presence of ASC was identified using liquid chromatography coupled to mass spectrometry (LC–MS). An ACQUITY Premier Ultra Performance Liquid Chromatograph (UPLC) coupled to a Synapt XS mass spectrometer (Waters) was used for the analysis. Enzyme reactions were diluted 20-fold with a 1:1 (v/v) mixture of deionized water and acetonitrile. Then, 1 µl of the diluted sample was injected onto a Z-HILIC column (1.7 µm, 95 Å pore, 2.1 mm × 150 mm, Waters). The mobile phases consisted of 30% (v/v) acetonitrile (A) and 95% (v/v) acetonitrile (B), both containing 0.1% ammonium hydroxide. The elution gradient was as follows: initial, 85% B for 5 min; linear gradient, 85% B to 45% B; isocratic, 45% B for 5 min; return to initial, 45% B to 85% B and re-equilibration, 85% B for 5 min. The flow rate was maintained at 0.2 ml min–1 throughout the analysis. The mass spectrometer was operated in negative ion mode with the following settings: capillary voltage: 2 kV, cone voltage: 25 V, m/z range: 100–1,500, scan cycle: 0.5 s and collision energy: 4 V. Lock mass correction was applied every 30 s using the peptide standard leucine-enkephalin at 100 pg µl–1 (Waters) with a mass window of 0.5 Da.
Anaerobic experiments
As previously described for LPMOs38, a 1 g l–1 suspension of Avicel PH-101 (Sigma Aldrich) in 50 mM sodium acetate buffer (pH 5.0) was prepared in a reaction glass vial and deoxygenated by flushing with nitrogen gas for 5 min under magnetic stirring. Solutions of 50 mM ASC, 10 mM hydrogen peroxide and 50 µM CelOCE, along with a water control, were deoxygenated using a Schlenk line (three cycles of 10 min vacuum and 2 min N2). All solutions were then placed in a Whitley DG250 anaerobic workstation for 16 h to ensure complete removal of oxygen. To initiate the reactions, 1 µM CelOCE was added to both anaerobic and aerobic Avicel suspensions. After 20 min of incubation, 100 µM hydrogen peroxide was added to half of the anaerobic reactions, whereas the remaining anaerobic reactions and all aerobic reactions received an equivalent volume of water. CelOCE activity was then triggered in all reaction mixtures by the addition of 1 mM ASC (final reaction volume: 600 µl in 2 ml tubes). Time-dependent reactions were conducted at 0, 1, 2, 3, 4, 8 and 16 h. Aerobic reactions served as positive controls to verify that the treatment of the stock solutions did not compromise reactant integrity. Reactions were terminated by boiling, followed by centrifugation at 21,000g for 15 min. The resulting supernatants were analysed by HPAEC–PAD. Each assay consisted of at least three independent experiments.
Hydrogen peroxide detection and quantification
Hydrogen peroxide production was evaluated using an assay adapted from a previous study71 originally designed for LPMOs. To measure the hydrogen peroxide production rate in the absence of a polysaccharide substrate, 1 µM CelOCE was mixed with 50 µM Amplex Red reagent (Invitrogen) and 5 U ml–1 horseradish peroxidase type II (HRP, Sigma Aldrich), in 50 mM sodium phosphate buffer pH 6.0, in a 96-well microplate and incubated at 37 °C in a spectrophotometer. After 2 min, 1 mM ASC (Sigma Aldrich) was added to initiate the reaction. Absorbance at 563 nm was measured every 5 min for a total of 30 min. To measure the change in hydrogen peroxide production in the presence of increasing concentrations of polysaccharide, 1 µM enzyme (wild-type CelOCE, CelOCE(Δ2–5) or TtAA9J) was mixed with 50 mM sodium phosphate buffer pH 6.0 and 0–100 g l–1 Avicel. Then, 1 mM ASC was added to initiate the reaction (final volume of 200 µl in 2 ml tubes). Incubation was performed in a ThermoMixer C (Eppendorf) at 37 °C and 850 rpm for 16 h (wild-type and mutant CelOCE enzymes) or 2 h (TtAA9J). After incubation, the reaction tubes were centrifuged at 20,000g for 10 min to separate the substrate. Then, 50 µl of the supernatant was added to 50 µl of a mix containing Amplex Red, HRP and sodium phosphate buffer pH 6.0, and measured as in the previous assay. Hydrogen peroxide produced during the reactions was quantified using a standard curve. Each assay consisted of at least three independent experiments.
Peroxidase activity and thermal stability of AA9 enzymes
Peroxidase activity was measured following a protocol described previously72. In brief, 1 mM of 2,6-dimethoxyphenol was mixed with 100 µM of hydrogen peroxide and 2–4 µM of AA9 in 50 mM sodium citrate buffer pH 5.0. The reactions were incubated in a ThermoMixer C at 50 °C and 850 rpm for 10, 20 and 30 min to ensure the linear phase of the activity. Immediately, the samples were transferred to a 96-well plate and the absorbance was measured at 469 nm in a spectrophotometer (Infinite M200 Pro, Tecan) using the i-Control v.1.10.4.0 software. Only the linear phase was considered to calculate the specific activity of each AA9. The quantification was done using the extinction coefficient of coerulignone (ɛ469 nm = 53,200 M−1 cm−1). For the thermal stability measurements, the stock solutions of 40 µM of each AA9 enzyme were incubated in a ThermoMixer C at 50 °C in 50 mM sodium citrate pH 5.0 for 6, 12, 24, 48 and 72 h. The peroxidase activity was measured at 30 °C in 50 mM Tris-HCl pH 7.5 for 15 min incubated in a spectrophotometer (Infinite M200 Pro, Tecan).
Complementary assays
Saccharification experiments were conducted to evaluate the ability of CelOCE to enhance the efficiency of the T. reesei Br_TrR03 enzyme cocktail27,28. Avicel, PASC and steam-exploded sugarcane bagasse were used as substrates. Reactions were performed in 2 ml microtubes with a final volume of 1 ml, using total solid contents ranging from 0.1 to 5% (w/v). Each reaction contained 50 mM sodium acetate buffer (pH 5.0), 2 µg enzyme cocktail and 50 µg CelOCE. Whereas ASC (1 mM) was included for Avicel and PASC reactions, it was excluded from those using sugarcane bagasse. Reactions were incubated at 40 °C for 24–72 h in a combi-D24 hybridization incubator (FinePCR), following the same saccharification assay protocol used for the enzyme cocktail. The cooperative effect of CelOCE with individual purified endo- and exo-enzymes was assessed using Avicel and PASC as substrates. Control reactions lacking enzyme, reductant (ASC) or both were also included. These reactions were incubated overnight at 37 °C and 850 rpm in a Thermomixer Comfort (Eppendorf). All reactions were terminated by boiling at 95 °C for 10 min, followed by centrifugation at 20,000g for 15 min. The supernatants were analysed by HPAEC–PAD and the boosting effect of CelOCE was determined by measuring the TRS released using the DNS method64. Complementary assays consisted of at least three independent experiments.
Turnover rate calculation
For a standard reaction, 0.1% (w/v) Avicel was incubated with 1 µM CelOCE and 1 mM ASC in 50 mM sodium acetate buffer (pH 5.0) at 37 °C for 0, 1, 2, 3, 4, 8 and 16 h. The apparent turnover rate was calculated on the basis of the linear correlation between cellobionic acid concentration (µM) and time (h) in the 1- to 4-h time frame, as described previously37.
Biophysical approaches for copper characterization
XRF and XAS
CelOCE in 20 mM HEPES buffer pH 7.4 (control) was incubated with 1 mM CuSO4 or with 1 mM NiSO4 (4 h) followed by the addition of 1 mM CuSO4 overnight. A final concentration of 1.4 mM CelOCE was loaded into a MicroRT Capillary (MiTeGen) for measurements. The XRF and XAS experiments were performed at the Extreme condition Methods of Analysis (EMA) beamline from the Brazilian Synchrotron Light Laboratory (LNLS/CNPEM) using a Vortex-ME4 detector (Hitachi). Data collection was performed using EMA control software and included energy steps of 0.5 keV and an acquisition time of 1 s per scan. The energy of the beamline was calibrated using the \({k}_{{a}_{1}}\) emission line and the first inflection point of a copper foil, while an ionization chamber monitored the incident beam intensity. The foil was positioned upstream of the sample and measured under the same conditions as CelOCE, with the first inflection point set to 9.074 keV. The spectra of all datasets were calibrated, normalized and merged using Athena73 software v.0.9.26.
EPR
CelOCE samples (0.5 mM) were prepared in 20 mM HEPES buffer (pH 6.5). EPR spectra were recorded on a Bruker Elexsys E580 spectrometer (Bruker) operating at X-band (9.14 GHz) at 100 K (BVT 3000 digital temperature controller) with the following acquisition parameters: modulation frequency, 100 kHz; modulation amplitude, 5 G; conversion time, 90 ms; sweep time, 92.1 s; and microwave power, 20 mW. Data acquisition was performed using the Xepr v.2.6b.119 software. EPR spin-Hamiltonian parameters were determined using a set of computational tools in two steps as follows. Initial parameter estimation: g and A tensors were estimated using laboratory-developed scripts in Python (SciPy/NumPy)74. The g‖ and A‖ values were inferred by analysing the singularities near the low-field edge of the spectrum (260–310 mT). Near the high-field edge (310–330 mT), the hyperfine splitting A⟂ was not resolved, so the average distance between the most intense peaks and shoulders was used to estimate the hyperfine couplings. The g⟂ value was determined from the central position between these peaks (Supplementary Table 9). Simulation and optimization: using these initial guesses, simulations were conducted with the Pepper module of the EasySpin 6.0.0 toolbox75, running in MATLAB software76 (MathWorks, v.9.13.0, R2022b). Diagonal components of the g and A tensors were allowed to vary independently in specified bounds during the fit. A global optimization was first performed using a genetic algorithm (25 generations), followed by local optimization with the Nelder–Mead downhill simplex algorithm. The final Hamiltonian parameters were obtained by second-order perturbation and exact diagonalization for the final simulations.
ITC
ITC experiments were conducted using a MicroCal PEAQ-ITC Automated system (Malvern Panalytical) and the PEAQ-ITC control v.1.50 software. CelOCE samples (100 µM) in 20 mM MES buffer (pH 6.5), treated with Chelex (Sigma-Aldrich) to remove trace metals, were placed in the reaction cell (200 µl volume). Either 1.0 mM CuCl2 or 1 mM cellobiose was loaded into the ITC syringe. Titrations were performed by injecting 2 µl aliquots into the reaction cell at 150 s intervals with a stirring speed of 500 rpm, for a total of 19 injections. ITC data were collected automatically using the MicroCal PEAQ-ITC automated control software and corrected for the heat of dilution by subtracting the heat generated by titrating the ligand into buffer alone. A single-site binding model was fitted to the data using the non-linear least-squares algorithm provided by the MicroCal PEAQ-ITC Automated analysis software. The fitting yielded the stoichiometry (n), dissociation constant (Kd) and enthalpy change (ΔH) of the reaction. Errors in ΔH, Kd and Gibbs free energy change (ΔG) were calculated as the s.d. for at least three independent experiments. Error in entropy change (ΔS) was determined through error propagation.
Structural and computational biology approaches
Crystallization, X-ray diffraction and structure determination and refinement
Crystals of CelOCE were obtained by vapour diffusion in solutions containing: 20% (w/v) polyethylene glycol (PEG) 8000, 3% (v/v) 2-methyl-2,4-pentanediol and 0.1 M imidazole, pH 6.5 (structure 1); 1.4 M trisodium citrate and 0.1 M HEPES buffer pH 7.5 (structure 2); or 20% PEG 6000, 0.1 M citric acid pH 3.0 and 5% glycerol (structure 3). The second crystal (structure 2) was cryoprotected in a solution containing the mother liquor added by 25% (v/v) glycerol for data collection. All crystals were collected and then flash cooled in liquid nitrogen. X-ray diffraction data were collected at 100 K on the MANACA beamline (LNLS-Sirius/CNPEM) using a Pilatus 2 M detector (Dectris) and the fine ϕ-slicing approach77. Diffraction data were obtained using the MxCuBE78 v.2 program. Three datasets, each comprising 3,600 images with a 0.1° rotation and 0.1 s exposure time, were collected. Data processing was performed using XDS79 (version of 30 June 2023, build 20230630). The structures were solved by molecular replacement with Phaser80 v.2.7.0, using a RoseTTAFold (v.1)-generated model81 as a search model. The initial model was refined using Phenix.Refine82 v.1.8.3 and manually adjusted in Coot83 v.0.8.9. The final model and metal coordination were verified using MolProbity84,85 v.4.5 and the CheckMyMetal86 v.2.1, respectively. Dimeric stability and interface analysis were assessed using PDBePISA87 v.1.52.
Molecular docking and computational simulations
The KVFinder88 v.1.1.1 software was used to identify the cavity corresponding to the CelOCE active site. A cellotetraose (C4) molecule, obtained from PDB entry 3WDY, was docked into the CelOCE structure using Autodock Vina72 v.1.1.2. Docking trials were performed in a 10 × 10 × 10 Å3 box centred on the copper region of the CelOCE monomer. To refine the ligand position in the active site, the docked structure was further relaxed, considering the functional dimer of CelOCE. In this set-up, one protomer was docked with cellotetraose, whereas the other remained ligand-free. Residue protonation states were assigned on the basis of a pH of 5.5, with H5 and H85 protonated at Nε, and H44, H46, H83 and H84 protonated at Nδ in both protomers. The system was solvated with TIP3P water molecules, neutralized with two sodium ions and minimized to eliminate steric clashes. Subsequently, the system was heated in four 1-ns steps to 300 K under the NVT ensemble, followed by a 10-ns equilibration under the NpT ensemble (T = 300 K, p = 1 bar). Position restraints were applied to protein, ligand and copper atoms during the initial minimization and heating steps. Distance restraints, maintaining the octahedral copper coordination with the N atoms of H44, H46 and H84, the O atom of Q50, and the O2 and O3 atoms of the second (−1) and third (+1) non-reducing end units of the ligand, respectively, were used throughout the remaining simulation steps. Simulations were performed using the Amber20 package, and structural and trajectory analyses were conducted with visual molecular dynamics (VMD)73. Protein structure images were generated with PyMOL v.2.3 (The PyMOL Molecular Graphics System).
Genetic engineering and characterization of T.
reesei
Integration of CelOCE and LsAA9A into T.
reesei Br_TrR03 strain
The T. reesei strain Br_TrR03 was engineered to express CelOCE and LsAA9A (an AA9 LPMO from L. similis) using a customized CRISPR–Cas9 approach as described in our previous work27. The genes encoding CelOCE and LsAA9A were optimized for codon usage in Trichoderma and integrated into the xyn4 and xyn5 loci, respectively. The 20-nucleotide protospacers designed to specifically target xyn4 (GCCAAACATACAGACTGAGT) and xyn5 (GCCTGCTCTCTGTCTACGGC) in T. reesei flanked by (5′-end) hammerhead (HH) and (3′-end) hepatitis delta virus (HDV) ribozyme sequences were inserted into the Bsp1407I-digested pTrCas9gRNA1 plasmid, which was further used in protoplast transformation. A markerless donor cassette was assembled in vivo by Saccharomyces cerevisiae, containing 1-kb flanking sequences homologous to regions upstream and 1-kb flanking sequences homologous to downstream targeted genome regions. The cassette was PCR amplified from the plasmid to generate linear DNA fragments, which were then used for fungal transformation. Obtained PCR products were purified and concentrated in a SpeedVac concentrator before use in transformation assays. The oligonucleotides used are listed in Supplementary Table 18. Then, T. reesei was transformed through protoplast-mediated transformation27,89. In each transformation event, 5 μg of the appropriate CRISPR–Cas9 vector and 5 μg of a linear DNA fragment for genomic integration were used. The genetic modifications to the genome of T. reesei strain Br_TrR03 were confirmed by PCR using different combinations of primers that anneal (upstream or downstream) of the targeted genome regions or internally to each integration cassette (Supplementary Table 18).
Shake-flask cultivation
Engineered T. reesei strains were grown in 250-ml Erlenmeyer flasks containing 50 ml of medium that comprised 20.0 g l–1 (NH4)2SO4, 20.0 g l–1 whole yeast cells (dry mass), 50 g l–1 of TRS from sugarcane molasses and pH adjusted to 4.8. The whole yeast cells were generated as described previously27. Sugarcane molasses (Mellaço de Cana) was diluted with water to a TRS concentration of approximately 350 g kg–1 and autoclaved separately before addition to the sterilized base medium. Each flask was inoculated with 0.3 ml of 107 spores per ml and cultivations were carried out in shaker incubators at 28 °C with 200 rpm for 5 days. After this period, samples were collected, centrifuged at 14,000g for 10 min at 4 °C and the supernatants were stored at −20 °C until analysis.
Bench-scale bioreactor cultivation
Bioreactor experiments were conducted using a BioFlo/CelliGen 115 system (Eppendorf) and water-jacketed 3.0 l vessels with a working volume of 1.0–1.7 l (ref. 28). The medium composition was identical to that used in shake-flask experiments, with the addition of 1.0 ml l–1 of J647 antifoaming agent (Struktol). The initial volume in the bioreactors was 1.0 l, including the 10% (v/v) inoculum. The inoculum was obtained by growing fungal spores in flasks, which were shaken as described above for four days at 28 °C and 200 rpm. The pH was maintained at 4.5 ± 0.5 using a solution of 2 M phosphoric acid and 10% (v/v) ammonium hydroxide. The temperature and aeration were maintained at 28.0 °C and 0.7 slpm compressed air, respectively. An agitation cascade (400–1,000 rpm) was used to ensure that the dissolved oxygen remained above 20%. A sugarcane molasses solution (approximately 350 g kg–1 of TRS) containing 1.0 ml l–1 of antifoaming agent was fed from 25 h of cultivation until 1 h before the end of the experiments at a feeding rate of 1.3 gTRS kg–1 h–1 in relation to the instant mass in the bioreactor, generating a non-linear feeding profile. During cultivation, samples were collected at regular intervals (24 h), centrifuged at 14,000g for 10 min at 4 °C and the supernatants stored at −20 °C for subsequent analysis. Final fermentation samples were obtained for hydrolysis experiments, protein quantification and enzymatic activity analysis.
Pilot plant scale bioreactor cultivation
The Trichoderma strain expressing CelOCE was cultivated in both 65-l (Bioflo 610, Eppendorf) and 300-l (Bioflo Pro 300 L, Eppendorf) pilot plant bioreactors. The composition of the molasses-based medium, as well as the cultivation and feeding conditions, were identical to those used in the bench-scale experiments. For the 65-l bioreactors, aeration was set to 13–18.5 slpm, dissolved oxygen 20%, pressure 5–10 psi and agitation 150–427 rpm. In the 300-l bioreactors, aeration was 84–120 slpm, dissolved oxygen 20%, pressure 5–10 psi and agitation 100–450 rpm. In both bioreactors, the inoculum volume was 10% of the total working volume.
Secretome analysis by mass spectrometry
Proteins (200 µg) from the secretome of engineered T. reesei strains were precipitated with acetone and resuspended in 500 µl of 25 mM ammonium bicarbonate buffer (pH 7.8) containing 50 mM dithiothreitol and 5% (w/v) sodium deoxycholate. After incubation at 60 °C for 30 min with agitation, samples were transferred to a 30 kDa MWCO Amicon filter (Merck Millipore) and centrifuged (14,000g, 20 min, 20 °C). The flowthrough was discarded and 450 µl of buffer containing 8 M urea was added, repeating this step twice. After the final centrifugation, 450 µl of 20 mM iodoacetamide in buffer was added for alkylation (45 min, in the dark at room temperature), followed by centrifugation and five desalting washes with buffer. Trypsin (1:30 w/w ratio) was added for overnight digestion at 37 °C. After centrifugation, peptides were collected in the flowthrough, washed twice with deionized water and dried. An internal standard (digested yeast alcohol dehydrogenase, P00330) was added, and 25 fmol of the peptide mixture was injected for LC–MS/MS analysis on a Synapt XS (Waters) coupled to an ACQUITY Premier UPLC. Data acquisitions were performed using the MassLynx v.4.2 program. Separation was performed on a peptide CSH column (1 mm × 100 mm, 1.7 µm, 130 Å) using a 4–55% gradient of over 103 min at 25 µl min–1. Data acquisition was in high-definition data-independent acquisition mode (50–2,000 m/z, 0.5 s scan cycle), with low collision energy at 6 eV and a ramp from 15 to 40 eV for elevated collision energy. Lock mass correction with the peptide standard leucine-enkephalin at 100 pg µl–1 was applied every 30 s with a mass window of 0.5 Da. Data processing was carried out in Progenesis 4.2 with a 1% false discovery rate, 20 ppm MS1 error tolerance and automatic MS2 error adjustment. Carbamidomethylation of cysteine was set as a fixed modification, methionine oxidation as variable and one missed trypsin cleavage was allowed. The T. reesei Br_TrR03 genome (NCBI: PRJNA1031947), including the CelOCE and LsAA9A sequences, served as the reference. Protein abundance was quantified using the top three peptides approach90.
Saccharification assays and secretome activity profiling
Enzymatic activities (β-glucosidase, β-xylosidase, endo-β-1,4-xylanase and CMCase) were determined as described previously27 and filter paper activity (FPase) was measured as described previously91. Saccharification reactions were conducted in 50 ml Nalgene Oak Ridge Centrifuge tubes using a combi-D24 hybridization incubator (FinePCR) at 50 °C with maximum rotation (level 9). Biomass pH was adjusted to 5.0 with 5 M NaOH. Each reaction contained 20.0 g total mass with 15% (w/w) final solids loaded in 100 mM acetate buffer (pH 5.0). Two stainless steel spheres (3.5 mm diameter, 1 g each) were added to ensure homogeneity. Distilled water replaced the enzyme in the blank reaction. Sampling and analysis followed an established protocol27. Three independent experiments were performed, and samples were collected every 24 h. Released sugars were quantified by HPLC (Agilent 1260 Infinity) with a refractive index detector. One-way ANOVA with post hoc Tukey’s honest significant difference tests were used to compare mean values of enzymatic activities and protein and sugar concentrations.
Inclusion and ethics statement
All researchers that fulfilled the authorship criteria by Nature Portfolio journals have been included in the author list. Their contributions were essential to the design, execution and interpretation of the study. The roles and responsibilities of each collaborator were clearly defined and mutually agreed ahead of the research. This research faced no severe restrictions or prohibitions in the setting of the researchers and was conducted in a manner that avoids causing stigmatization, incrimination, discrimination or personal risk to any parties involved. We support inclusive, diverse and equitable conduct of research.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.