Sediment sampling
For sampling of living benthic foraminifera, samples were taken during various surveys at Sagami Bay (Japan), the Rainbow Vent Field at the MAR, the Bedford Basin (Canada) and the German Wadden Sea (Friedrichskoog). Samples at Sagami Bay were taken during a RV Kaimei cruise in September 2019 and on RV Yokosuka cruises in October 2022 and May 2023. Sediment was retrieved using a multicorer during the former and a push core during the latter at stations in central Sagamy Bay (NSB site; Supplementary Table ST2). Samples from the region around the Rainbow Vent Field were retrieved during RV Meteor cruise no. M176/2 in September 2021, using a multicorer at six stations (Supplementary Table ST2). Samples from the Bedford Basin were retrieved in March 2022 on board the dive vessel EastCom, using a multicorer at three stations (Supplementary Table ST2). Sediments from the intertidal mudflats in Friedrichskoog were retrieved manually at one station in November 2021 and May 2023. The top 1 cm of sediment was scraped off by spoon. Surface sediments from the brackish water salt marsh of Hirakata Bay, Yokohama (Japan) were collected in 2015 (Supplementary Table ST2), and isolated A. veneta strains were maintained in the laboratory.
Preparation of living foraminifera for intracellular phosphate analyses
The top 1 cm of sediment was directly wet sieved over a 125 or 250-µm mesh within a time frame of 2 h following core retrieval, using either filtered regional surface water or nitrate- and phosphate-free artificial seawater (ASW) at ambient salinity prepared using Red Sea Salt. Only certain samples from the Bedford Basin were sieved within 2 days following sampling, using filtered seawater from the Bedford Basin. Sediments retrieved from Friedrichskoog in March 2023 were directly sieved in the field, using surface water.
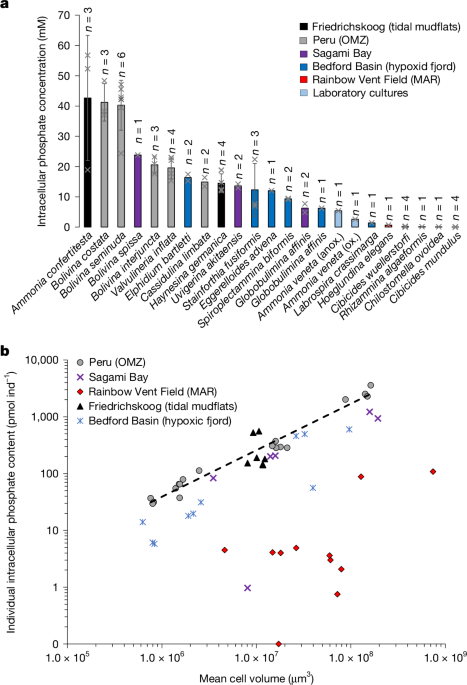
Living foraminifera specimens for intracellular phosphate analyses were wet picked from the coarse residue (over 250 µm for Friedrichskoog samples and over 125 µm for the remainder). In total, 34 samples were picked that included 15 different benthic foraminifera species. Specimens of A. veneta were obtained from the clonal strain cultured at the Japan Agency for Marine-Earth Science and Technology (JAMSTEC). For a description of culturing methods, see below. One A. veneta sample was prepared from oxic incubation and one from anoxic. Each foraminiferal sample contained between one and 75 living foraminifera specimens depending on the average size of the species (Supplementary Table ST2). Note that the sample of R. algaeformis contained only one large fragment of the organism, which was visibly filled with cytoplasm. All samples were photographed with a stereomicroscopic camera for subsequent determination of foraminiferal biovolume. Afterwards, foraminifera were cleaned and phosphate extracted using the methods described in ref. 1. Specimens were rinsed with phosphate-free ASW, prepared from Red Sea Salt, then transferred to centrifuge tubes with the lowest amount of ASW possible. Next, 3 ml of reverse osmosis water (with conductivity of 0.055 µS cm−1) was added to samples. Within the water, foraminiferal specimens were broken up using a clean pipette tip. A procedural blank underwent the same procedure without foraminiferal specimens, for blank corrections of NO3− analyses (13 procedural blanks in total). All samples were frozen at −20 °C for at least 2 h and subsequently thawed. This procedure was repeated three times. Freeze–thaw injuries damage plasma membranes and increase their permeability61,62. Subsequently, samples were filtered through sterile 0.2-μm cellulose acetate filters.
A slightly adapted protocol was used for the extraction of P compounds for 31P-NMR; these samples were retrieved from Friedrichskoog in March 2023. Two replicates were picked, each containing around 1,000 living specimens of A. confertitesta. Specimens were rinsed with phosphate-free ASW prepared from Red Sea Salt and subsequently transferred to microcentrifuge tubes with the lowest amount of ASW possible. Next, 600 µl of heavy water (D2O) with 1 M KOH was added to samples. Within the water, foraminiferal specimens were broken up using a clean pipette tip. Samples were frozen at −20 °C for at least 2 h and then thawed. This procedure was repeated three times. Subsequently, samples were centrifuged. A further sample was picked that contained roughly 1,500 specimens of A. confertitesta, which were transferred unharmed to a NMR tube containing D2O and with a salinity of 28.
Analyses of extracted intracellular phosphate
Filtered samples were analysed for total dissolved phosphate by segmented flow-injection analysis using a QUAATRO39 (Seal Analytical) autoanalyser, which included a XY2-autosampler unit, at GEOMAR Helmholtz Centre for Ocean Research Kiel (Kiel, Germany). Samples from the MAR were analysed, using the same methods, directly on board during RV Meteor cruise no. M176/2. The system set-up included four channels—for nitrate + nitrite, silicate, nitrite and phosphate—but only the phosphate data were used within this study. The method used for phosphate analysis corresponds to Q-064-05 Rev. 8 (developed by Nederlands Instituut voor Onderzoek der Zee; detection limit 0.004 µmol l−1 and described by QuAAtro Applications).
Biovolumetric determination of living foraminifera
Total foraminiferal cell volume of each species was estimated following previously published methods63. We assumed that internal test volume corresponds to 75% of total test volume and is completely filled with cytoplasm64. Methodology and equations used for precise biovolume estimation in several benthic foraminifera species are given in ref. 65 but, unfortunately, none of the species analysed in our investigation are listed in their study; therefore, the closest geometric shape was used for biovolume estimation (Supplementary Table ST3). In total, the biovolume of 850 foraminiferal specimens was determined.
Note that, for the two shapes related to B. spissa (cone with elliptic base) and C. wuellerstorfi (triaxial ellipsoid), the heights of the specimens would have been required, which were not visible on the images. The following approximations have been used instead: for B. spissa specimens, height was estimated by the previously determined mean height of the species (133 ± 7 μm)16; for C. wuellerstorfi, the average ratio of the shortest diameter on the spiral side to the height was determined (0.424 ± 0.029, 1 s.e.), using published images of the species66 (Supplementary Table ST5).
Determination of living abundance
One sample retrieved from Friedrichskoog in March 2023 was taken to determine the abundance and population density of A. confertitesta. For this sample, we used a square-shaped metal frame with a side length of 10 cm. Within this metal frame, the top 1 cm of sediment was scraped off and collected in a polyethylene bottle. Next, a mixture of ethanol and Rose Bengal (2 g l−1) was added to the bottle until ethanol concentration exceeded 70%. The jar caps were cleaned and applied tightly, the height of the sediment in the jar was marked and the jars stored for at least 14 days at room temperature until further analysis. Subsequently, stained samples were wet sieved over a 125-µm mesh, dried at around 40 °C and the jars filled with water up to the sediment fill mark level. The volume of water representing bulk sediment volume was measured in a graduated cylinder (approximate accuracy ±5 cm3). Subsequently, samples were split using a dry splitter, and specimens of A. confertitesta stained with vital raspberry red were counted under the microscope. Living stained foraminifera were fixed in plummer cells.
Culture of A. veneta
Cultures of A. veneta were from the same strain used by Ishitani et al.17 isolated first in 2015. Specimens were cultured in ASW at a salinity of 35, at 23 °C under 14/10 h light/dark cycles. The specimens used for this experiment were fed, frozen, dead Dunaliella salina (no. NIES-2257). We isolated five specimens with shell diameter 150–300 μm from subculture into 35-mm culture dishes with 5 ml of ASW, with culture for 4 days under both oxic and anoxic conditions. We cultured normally for oxic conditions in an AnaeroPack-Anaero, which can maintain 0% O2 and 15% CO2 for anoxic conditions.
Comparative genomics and metabarcoding
Creatine kinase homologues were identified with the KEGG KAAS tool67 (species: hsa, mmu, rno, dre, dme, cel, ath, sce, ago, cal, spo, ecu, pfa, cho, ehi, eco, nme, hpy, bsu, lla, mge, mtu, syn, aae, mja, ape, mbr, ddi, tet, smin, pti, ehx, gtt, ngr, tva, tbr, spar) as K00933. The following genomes and transcriptomes were screened for comparative genomic analysis:
Subsequently, published transcriptome data of Ammonia spp. were collected from the NCBI database for further identification of this taxon at species level by metabarcoding. Collected raw reads were quality filtered with FASTX-Toolkit 0.0.13 (ref. 69), and those with fewer than 50 bases—or that included ambiguous barcodes and showed poor quality (q-score <20)—were removed.
NMR spectroscopy
All NMR experiments were carried out on a Bruker 600 MHz Avance III HD spectrometer (14.09 T, 600.13 MHz for 1H, and 242.94 MHz for 31P) at 298 K in D2O. One-dimensional 31P{1H} spectra were obtained utilizing a 30° excitation pulse and relaxation delay of 1.0 s. The waltz16 sequence was implemented for proton decoupling. Spectra were acquired at a spectral width of 96,153.84 Hz and 65,536 time domain data points, by recording 1,024 scans for extracted samples and 3,584 for the sample containing live foraminiferal cells31. P chemical shifts were referenced to external phosphoric acid (external measurement). Data were acquired using TopSpin v.3.6.4, and all spectra were processed with Topspin v.4.1.4, applying zero filling and an exponential multiplication of the free induction decay with a line-broadening factor of 1.0 Hz.
Cryofixation for cryo-SEM–EDS
Bolivina spissa specimens were isolated from the topmost 1 cm of sediment directly onboard, immediately following sampling under a stereomicroscope; A. veneta specimens were picked from cultures. Cryofixation followed the protocols of ref. 70. For cryofixation of foraminifera, conductive glue comprising 30 wt% graphite oxide and glycerol was used with probe sonication, following the method described in ref. 71 with solvent modification. Glycerol was applied as a cryoprotectant and viscous dispersant to prevent sinking of foraminifera deep into the glue. The glue was pasted onto an aluminium rivet (diameter 3 mm), each foraminifer specimen was mounted on the glue using an eyelash brush and was then immediately frozen in semifrozen isopentane at −159.8 °C. The rivet was then mounted on an ultramicrotome (Ultracut S equipped with FCS, Leica Microsystems, operated at −130 °C) and the cross-section faced using a diamond knife (Diatome). Faced samples were stored in a container below −160 °C until required for cryo-SEM observation.
Cryo-SEM–EDS
In total, three B. spissa specimens (two from the October 2022 cruise and one from the May 2023 cruise) and eight A. veneta specimens were analysed. Cryo-SEM observation was performed on a Helios G4 UX (Thermo Fisher Scientific) equipped with a cryogenic stage and a cryopreparation chamber (catalogue no. PP3010T, Quorum Technologies). EDS analyses were performed on an Octane Elite Super (C5) (AMETEK), which was attached to the cryo-SEM (software TeamEDS, v.4.6.0052.0238). The sample was mounted on a transfer shuttle in liquid nitrogen, then vacuum transferred to the cryopreparation chamber. Water was sublimed at −80 °C for 8 min to expose the organelle structure, and Cr was then coated by magnetron sputtering at 20 mA for 60 s. Note that sublimation does not melt or sublime glycerol-based glue. We selected Cr because conventional Pt or Au sputtering causes overlap in EDS signals, including P; the K-lines of N and P appear at 0.392 and 2.013 kV, respectively, and the M-lines of Pt and Au at 2.048 and 2.120 keV, respectively. By contrast, the L-line of Cr appears at 0.573 eV, overlapping only with O, and no signals appear between it and its K-line at 5.414 keV. The sputtered sample was transferred to the SEM chamber and maintained below −140 °C; cross-sectional morphology was imaged by secondary electron at 2 kV with 50 V of antibias on the sample, and EDS mapping was performed at 20 kV without antibias.
Spectral treatment aiming to deconvolve signal from noise was performed on EDS elemental maps. Conventional quantitative EDS analyses use correction by atomic number (Z), absorption (A) and X-ray fluorescence (F), called the ZAF method, assuming that the surface is completely flat and that elemental composition, along with depth direction, is homogeneous. However, our cryo-SEM and cryo-EDS maps did not meet these requirements and the apparent EDS maps were correlated with background, so that we could see the similarity between count-per-second (CPS) maps and the EDS maps of low-intensity atoms. Therefore, we tried to suppress the position-dependent background signal72. EDS maps are generated from the number of counts in which X-rays from atom A appear, and count IA is composed of pure signal SA and background BA, where background is mostly due to the bremsstrahlung effect. Because CPS is the sum of signal from all atoms and background, \({\rm{CPS}}={\Sigma }_{i}{S}_{i}+B={\Sigma }_{i}{I}_{i}+{B}_{\bar{i}}\), where i indicates the atom of interest, B is the summation of background intensity for all of the energy and Bi is the summation of background intensity at energy ranges in which characteristic X-ray peaks are absent. The background mostly derives from bremsstrahlung and is thus dependent on beam condition, and here we assume that background shape is the same in any position of EDS maps. The intensity-to-noise ratio (R) of atom i at position (m, n) is defined as equation (1):
$$\begin{array}{c}R({\rm{m}},{\rm{n}})={I}_{i}({\rm{m}},{\rm{n}})/{B}_{i}({\rm{m}},{\rm{n}})={S}_{i}({\rm{m}},{\rm{n}})/{B}_{i}({\rm{m}},{\rm{n}})+1\\ \,\,\,\,=\,{I}_{i}({\rm{m}},{\rm{n}})/({\rm{CPS}}-\sum _{{\rm{i}}}{I}_{i}({\rm{m}},{\rm{n}}),\end{array}$$
(1)
which is related to conventional signal-to-noise ratio Si/Bi. We used R to emphasize localized minor elements hidden under the strong background.
Finally, colocalization of SEM images and the EDS elemental map was performed manually using calcium distribution maps, the main compound of the test in calcitic foraminifera. When needed, we performed EDS map scaling and/or rotation without deformation (that is, warping). For enhanced visualization of elemental distribution on EDS maps, a 16-colour look-up table was applied on EDS maps without grey value modification (Fig. 2c). Then, for all elements (excluding Ca), one-pixel median filtering was performed to smoothe the elemental distribution map and identify enriched ultrastructures regarding the element of interest. Image treatment was performed using the software Fiji73.
TEM–EDS
Foraminifera specimens were fixed with 2.5% glutaraldehyde in filtered ASW for at least 24 h at 4 °C. They were then embedded in 1% aqueous agarose and cut into cubes of roughly 1 mm3. Fixed specimens were embedded in 1% aqueous agarose, decalcified with 0.2% ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid in 0.81 mol l−1 aqueous sucrose solution (pH 7.0) for several days and then rinsed with filtered seawater. For measurement of P in cells with EDS, we did not conduct postfixation with osmium tetroxide, which was the overlapped energy peak position of P. Specimens embedded in agarose were rinsed with filtered ASW, stained with 2% uranyl acetate solution for 2 h at 4 °C, dehydrated in a graded ethanol series and embedded in epoxy resin (Quetol 651).
Ultrathin sections (100 nm) were cut using a diamond knife on an Ultracut S ultramicrotome and then stained with 2% aqueous uranyl acetate and lead staining solution (0.3% lead nitrate and 0.3% lead acetate, Sigma-Aldrich). TEM–EDS imaging was performed on a Tecnai G2 20 (Thermo Fisher Scientific), equipped with a bottom-mounted 2k × 2k Eagle charge-coupled device camera (Thermo Fisher Scientific) and a RTEM-S 61700ME EDS detector, (AMETEK) at an acceleration voltage of 200 kV. Note that the elements used for staining are heavier than Au and do not overlap with P.
Calculation of total phosphate storage in living foraminiferal assemblages
The total dissolved inorganic phosphate pool stored in foraminifera in the sediment column (∑DIPi sed., in mmol m−2) was calculated for locations in the Southern North Sea region around the Wadden Sea (from 2 to 10 °E and from 51 to 55 °N) and for the Peruvian continental margin (from 10 to 15 °S). Assemblage data for the North Sea region include 135 stations from the literature51,52,53,54,55,56,57 and one at Friedrichskoog (from this study). Assemblage data for Peru include 35 stations1,29.
The ∑DIPi sed. for 14 stations off Peru has previously been calculated, and is derived directly from ref. 1 For the remaining stations, ∑DIPi sed. was calculated according to equation (2) using the composition of benthic foraminiferal assemblages and intracellular phosphate content for each species (phosphatei n; Table 1):
$$\sum {{\rm{DIP}}}_{i\,{\rm{sed.}}}=\sum {A}_{n}\times {{\rm{phosphate}}}_{i\,n}\times {10}^{-9},$$
(2)
where An is the abundance (living) of foraminiferal species n (in ind m−2) and phosphatei n is the mean intracellular phosphate content of species n (in pmol ind−1). Individual phosphate storage data for A. veneta were not used for these calculations, because it was the only species for which no measurements were available from environmental samples (from laboratory cultures only). For species with unknown intracellular phosphate storage that share a genus with other species for which intracellular phosphate storage had already been determined, the average individual intracellular phosphate storage for this genus was used. Other species with unknown phosphate storage were excluded from the calculations. Finally, mmol m−2 was converted to g m−2 using the molar mass of phosphate (approximately 95 g mol−1). All assemblage data used for caclulations are summarized in Supplementary Table ST8 (Southern North Sea) and Supplementary Table ST9 (Peru) as downloadable spreadsheets.
Estimation of coastal riverine P runoff
Riverine phosphate runoff for the most important river estuaries of the Southern North Sea (Rhine, Meuse, Noordzeekanaal, Ijsselmeer, Ems, Weser and Elbe) was recorded for the year 2019 from the report of a monitoring programme58. Riverine total P runoff to the Peruvian coast from 10 to 15 °S, was taken from a global modelling study for the year 201559.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.