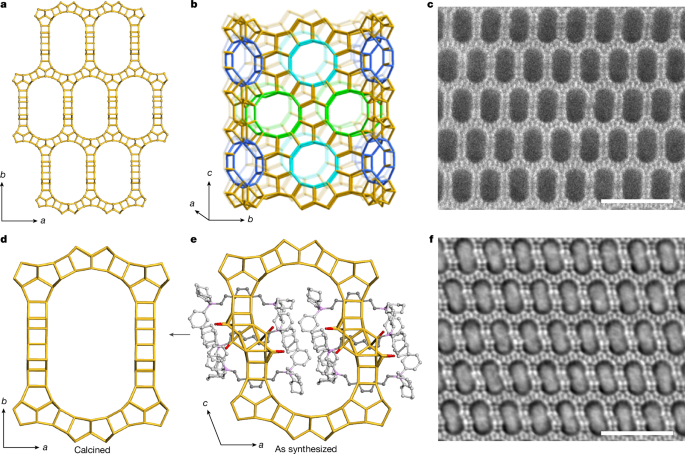
OSDA synthesis
The OSDA used in this work is a di-quaternary phosphonium cation (bis-1,8(tricyclohexyl phosphonium) octamethylene, denoted as Tri-Cy-dC8, consisting of tricyclohexylphosphonium head groups connected by a linear methylene chain with eight carbons (Extended Data Fig. 1a). The synthesis of Tri-Cy-dC8 was carried out by the reaction of tricyclohexylphosphine with corresponding linear dibromoalkanes, Br(CH2)8Br. In a typical synthesis, 36.514âg (0.125âmol) of tricyclohexylphosphine (Aladin, >96%) was dissolved in 150âml chloroform (Sinopharm, â¥99%) in a three-neck 500-ml flask immersed in an ice bath. The linear 1,8-dibromooctane (0.05âmol, Aladin, 98%) dissolved in 50âml chloroform was added dropwise into the flask through an addition funnel. The mixture was stirred for 2âh and then heated to reflux for 4âdays. After cooling to ambient temperature, excessive ethyl acetate was added to precipitate the white product, which was washed twice with ether and subjected to rotary evaporation to obtain the pure white OSDA dibromide salts. The structure and purity of the products were confirmed by liquid 1H and 13C NMR in D2O (Supplementary Figs. 1 and 2).
The dibromide salts were converted to their corresponding hydroxide forms by anion exchange using anion exchange resin (Xidian, 1.1 mequiv/1âml) in batch mode. The hydroxide solution was concentrated by rotary evaporation under vacuum. The concentration of the final solution was determined by titration with 0.1âN HCl (Beijing North Weiye Institute of Measuring and Testing Technology) using phenolphthalein as an indicator, and the weight percentage of the OSDA(OH)2 is generally about 30âwt%.
Zeolite syntheses
Synthesis in the hydroxide medium
ZMQ-1 zeolite was initially discovered in hydroxide-mediated synthesis at 180â°C with a gel molar composition of 1.0SiO2:0.02Al2O3:0.25Tri-Cy-dC8(OH)2:10H2O. A typical synthesis procedure was as follows. 0.458âg (0.0022âmol) of aluminium isopropoxide (Macklin, AR) was dissolved in the OSDA(OH)2 solution (0.0137âmol) in a plastic beaker under magnetic stirring, followed by 11.458âg (0.0549âmol) of tetraethyl orthosilicate (Sinopharm, â¥99%). The mixture was homogenized to obtain a clear sol, which was then hydrolysed at ambient temperature overnight to evaporate partial water and ethanol. The gel was then heated at 100â°C in a convection oven to remove residue water and ethanol to reach the target gel molar composition by weighting. The viscous gel was transferred into Teflon-lined autoclaves and heated at 180â°C in a convection oven statically for 10âdays. The solid product was recovered by centrifugation and washing three times with water (200âml), ethanol (100âml) and acetone (100âml), then dried at 100â°C for 12âh and subsequently calcined at 600â°C for 12âh with a ramping time of 6âh. The yield of the product is 98% based on SiO2.
The crystallization time could be shortened to 5âdays by increasing the crystallization temperature to 190â°C with the same gel composition following the procedure described above. Further, by varying the molar ratio of Al2O3, ZMQ-1 zeolites with different framework Si/Al ratios were successfully synthesized using the gel molar compositions of 1.0SiO2:0.01Al2O3:0.25Tri-Cy-dC8(OH)2:10H2O and 1.0SiO2:0.005Al2O3:0.25 Tri-Cy-dC8(OH)2:10H2O. The solid product was recovered and processed according to the procedures described above.
ZMQ-1 was also successfully obtained by using fumed silica (Macklin, AR) and aluminium sulfate octadecahydrate (Sinopharm, AR) as Si and Al sources with different H2O/SiO2 ratios under static and rotation crystallization modes. In a typical synthesis, a synthesis gel with molar composition of 1.0SiO2:0.02Al2O3:0.25Tri-Cy-dC8(OH)2:xH2O, in which xâ=â10, 16 and 30, was prepared and then subjected to heating in Teflon-lined autoclaves in a static or rotation oven for different durations. The product was collected and processed according to the procedures described above.
Synthesis in the fluoride medium
ZMQ-1 zeolite was obtained from the fluoride medium with a gel molar composition of 1.0SiO2:0.01Al2O3:0.25Tri-Cy-dC8(OH)2:0.5HF:10H2O. In a typical procedure, aluminosilicate gel was first prepared according to that of the synthesis in hydroxide medium at 180â°C. Then, 0.596âg (0.0275âmol) of HF (48%) was added to the gel and the resulting mixture was homogenized for 10âmin using a spatula. (Caution! This must be done in a fume hood because of the toxic and corrosive gases generated during the mixing process.) The obtained viscous gel was transferred into a Teflon-lined autoclave and heated at 190â°C in a convection oven statically for 10â15âdays. The solid product was washed and then calcined according to the procedures mentioned above. The yield of product is 96% based on SiO2.
Zeolite synthesis overview
The synthesis parameters and phases obtained are summarized in Supplementary Table 2. The first and foremost critical factor for the successful formation of ZMQ-1 phase is the introduction of Al, because pure silica syntheses in both hydroxide and fluoride media all produced unknown phase(s). Si/Al ratio in the synthetic gel ranging from 25 to 100 produced pure ZMQ-1 zeolites, but further increase and decrease of Si/Al directed to the unknown phase(s). Besides, heating temperature is vital for forming pure ZMQ-1 from aluminosilicate gel in hydroxide medium. Unknown phase(s) of little importance existed at a lower temperature of 180â°C, whereas they almost diminished at a higher temperature of 190â°C.
Removal of phosphorous species
Phosphorous species (oxides or phosphates) left in the calcined ZMQ-1 zeolites were removed by washing and ion exchanging with NH4Cl solution. Typically, 0.2âg of calcined zeolite powder was mixed with 10âg NH4Cl solution with a concentration of 1âmolâlâ1 in a sealed plastic bottle. The mixture was stirred in an oil bath at 80â°C overnight. The solid was collected by washing and filtration and then dried at 100â°C. The proton-form zeolite was obtained by calcining the above ammonium-form powder at 600â°C for 3âh with a 6-h ramp.
Thermal and hydrothermal stability test
The thermal stability of as-synthesized ZMQ-1 was tested in a muffle furnace at temperatures of 600â°C, 800â°C and 1,000â°C in air for 6âh, 1âh and 1âh, respectively, with a ramping rate of 6âh. The hydrothermal stability test was performed for the calcined zeolite (600â°C for 12âh) in a fixed-bed reactor at temperatures of 600â°C, 700â°C and 800â°C for 3âh with a relative humidity of 50% by purging deionized water with N2 flow. 0.1âg of zeolite powder was placed in a quartz reactor tube supported by quartz wool and then heated under N2 flow to the target temperature. Subsequently, deionized water was purged into the tube reactor by using a peristaltic pump to generate a mixture gas of N2 and steam. After the 3-h treatment finished, the system was cooled to room temperature and the zeolite powder was taken out for analysis.
27Al NMR shows that ZMQ-1 calcined at 600â°C and 800â°C have similar resonances centred at about 58.3, 41.9 and â9.7âppm (Supplementary Fig. 4a), which could be assigned to Al species in tetrahedrally coordinated framework sites, distorted tetrahedrally coordinated framework sites and octahedrally coordinated framework sites interacting with polymeric phosphate species34, respectively. By contrast, ZMQ-1 calcined at 1,000â°C shows the absence of resonance at about 58.3âppm, indicating the possible disappearance of tetrahedrally coordinated framework Al. The prominent resonance at about 40.6âppm is typically assigned to tetrahedrally coordinated Al in amorphous aluminophosphates formed by leached Al from the framework and P (ref.â35). Other resonances at 9.3 and â11.7âppm could be assigned to octahedrally coordinated Al in aluminophosphates36. Ar adsorptionâdesorption isotherms and pore size distribution of the three calcined ZMQ-1 zeolites show that pore structure was preserved for zeolites calcined at 600â°C and 800â°C but almost disappeared for the 1,000â°C counterpart (Supplementary Fig. 4bâd and Supplementary Table 5), indicating the collapse of the structure, most probably because of the dealumination as corroborated by 27Al NMR spectra (Supplementary Fig. 4a). The thermal and hydrothermal stability of phosphorus-free ZMQ-1 (ZMQ-1(CW)) were also evaluated. ZMQ-1(CW) shows higher thermal stability up to 1,000â°C and hydrothermal stability comparable with that of phosphorus-containing counterpart (Extended Data Fig. 8e,f). The N2 adsorptionâdesorption data of ZMQ-1(CW) calcined at different temperatures show decreased gas adsorption amounts and micropore volumes, along with elevated heating temperatures (Supplementary Fig. 5a and Supplementary Table 6).
General characterizations
Unless otherwise stated, the physicochemical characterizations have been performed for ZMQ-1 samples synthesized in hydroxide medium. Laboratory PXRD patterns of the zeolites were collected in a Rigaku LabView diffractometer (CuKα, λâ=â1.5418âà ). High-temperature in situ PXRD was carried out on the same diffractometer with a Reactor-X cell, which has a Si sample holder in a heated chamber with a window made of Be foil. PXRD patterns of selected samples were taken during a heating programme at 5â°Câminâ1 heating rate at 50â°C steps up to 1,000â°C. At each step, the temperature was held for 1âmin for PXRD recording. The morphology and size of zeolite crystals were examined using a Hitachi S-4800 scanning electron microscope equipped with a cold field-emission gun with an accelerating voltage of 2âkV. The technical details of the experimental set-up used for in situ FTIR analysis in this work have been described elsewhere28. Thermogravimetric analysis of the as-synthesized zeolites was performed using a Rigaku TG-DTA8122 thermal analyser system in 50âmlâminâ1 air flow with a heating rate of 10â°Câminâ1 from 30 to 1,000â°C. The textural properties of the zeolites after OSDA removal were investigated by physisorption of Ar at 87âK using a Micromeritics 3Flex instrument equipped with a cryostat I accessory. The samples were outgassed under vacuum at 350â°C for 12âh before measurement. Analysis parameters were carefully selected to ensure proper equilibration of the data. The apparent surface area was calculated using the BrunauerâEmmettâTeller method and following the procedure recommended in ref.â37. The cumulative pore volume and pore size distributions were calculated by applying the kernel of (metastable) NLDFT adsorption isotherms considering a zeolitic surface and isolated cylinders as a pore model. Although this model gives the best approximation available so far, validating the main mode pore sizes, it is evident that it does not perfectly describe the complex pore structure and potential surface roughness of the ZMQ-1 zeolite, that is, main elongated cylindrical-like pores interconnected by means of smaller cylindrical windows. Micropore volumes were also determined using the same kernel. The calculations were carried out using the VersaWin 1.0 software package provided by QuantaTec (Anton Paar). For comparison purposes, the zeolites were also analysed on a QuantaTec Autosorb iQ sorption analyser at 87âK. Statistically consistent results were obtained. Contents of Si, Al and P were analysed by inductively coupled plasma optical emission spectroscopy using an Elementar Unicube apparatus. We performed CHN analysis using a Thermo Fisher iCAP PRO analyser.
Solid-state NMR
29Si, 27Al and 31P MAS NMR spectra were recorded on a Bruker Avance III HD 500âMHz spectrometer operating at 11.7âT using 4.0-mm rotors spun at νMASâ=â14âkHz. The resonance frequencies of 29Si, 27Al and 31P were 99.4, 130.4 and 202.6âMHz, respectively. For 29Si MAS NMR, single-pulse duration of 2.33âµs corresponding to a flip angle of Ï/3 and a recycling delay of 20âs were used. 10,240 scans were acquired and chemical shifts were referenced towards teramethylsilane. For 27Al MAS NMR, single-pulse duration of 1.75âµs corresponding to a flip angle of Ï/12 and a recycling delay of 1âs were used. 4,096 scans were acquired and chemical shifts were referenced towards aluminium nitrate (Al(NO3)3). For 31P MAS NMR, single-pulse duration of 5.00âµs corresponding to a flip angle of Ï/2 and a recycling delay of 8âs were used. 1,600 scans were acquired and chemical shifts were referenced towards a saturated H3PO4 solution. 13C cross-polarization magic angle spinning (CPMAS) NMR spectra were recorded on a Bruker Avance III 600âMHz spectrometer at a resonance frequency of 150.9âMHz. 13C CPMAS NMR spectra were recorded using a 4-mm MAS probe and a spinning rate of 12âkHz. A contact time of 4âms and a recycle delay of 2âs were used for the 13C CPMAS NMR measurement. The chemical shifts of 13C was referenced to teramethylsilane.
STEM
Samples for the STEM imaging were prepared by embedding a small amount (about 5âμg) of ZMQ-1 in LR White Resin within a gelatin capsule (size 00). The capsule was then hardened at 60â°C for 24âh. To create ultrathin sections with an estimated thickness of 50ânm, a Leica Ultracut UCT Ultramicrotome equipped with a 45° diamond knife from DiATOME was used. The sections were then transferred to Lacey carbon-supported copper grids. iDPC-STEM and ADF-STEM images of ZMQ-1 were obtained using a double aberration-corrected Themis Z TEM (Thermo Fisher Scientific) operated at an accelerating voltage of 300 kV. The images were acquired using a beam current of 10âpA, a convergence angle of 16âmrad and a dwell time of 5âμs. The iDPC-STEM images were formed using a segmented annular detector. A high-pass filter was applied to the iDPC-STEM images to reduce low-frequency contrast.
3DâED
Sample preparation
The as-synthesized or calcined ZMQ-1 samples obtained by means of the hydroxide route were dispersed in acetone and then the suspensions were treated by ultrasonication for about 30âs. One droplet from the suspension was applied on a Lacey carbon-supported copper TEM grid for further cRED data collection.
Data collection
cRED data were collected at room temperature on a JEOL JEM-2100 transmission electron microscope (LaB6, 200âkV) using a high-speed Timepix hybrid camera (512âÃâ512 pixels, Amsterdam Scientific Instruments). The software Instamatic was used for the collection of cRED data. The crystals were rotated continuously at a rate of 0.45°âsâ1 during the data collection. The exposure time was 0.5âs per frame, which was integrated over 0.225° of reciprocal space. The electron flux density and camera length were 0.1âeââà â2âsâ1 and 25âcm, respectively.
To locate the OSDAs in the pores, low-dose cRED datasets were collected under cryogenic conditions on a 300-kV Titan Krios G2 equipped with a Ceta-D camera. The prepared TEM grids with the as-synthesized ZMQ-1 were plunge-frozen in liquid ethane and then transferred into the Krios through cryogenic transfer. To minimize the unnecessary electron exposure, EPU-D was used to ensure that the sample was exposed to the electron beam only during the data collection. A flux density of 0.0025âeââà â2âsâ1 was used, which gave a cumulative fluence of 0.375âeââà â2 per dataset with a goniometer rotation range of 60°.
Data processing and structure determination
Rotation electron diffraction processing software (REDp) was first used to process cRED datasets to determine the unit cell and space group38. With the obtained unit cell and space group, cRED datasets were further indexed using X-ray detector software (XDS) to extract the reflection information39. For calcined ZMQ-1, the structure was solved from a single dataset using SHELXT. For as-synthesized ZMQ-1, three room-temperature datasets were merged based on their cross-correlation to improve completeness and I/Ï(I). With the merged data, the average structure of as-synthesized ZMQ-1 was solved using SHELXT. The obtained structural models for both calcined and as-synthesized ZMQ-1 were further refined by SHELXL using the ShelXle GUI. All framework atoms were refined anisotropically. Soft restraints on bond distances and angles were applied in the refinement. Electron diffraction frames that show severe dynamical effects (that is, electron diffraction frames taken close to zone axes) were excluded during the data processing.
Treatment of positional disorder in structure refinement of as-synthesized ZMQ-1
During the structure refinement, positional disorder was found for two (Si29 and Si30) out of 30 symmetry-independent Si atoms. Two more Si peaks, assigned as Si31 and Si32, were located at a distance of 1.1 and 1.3âà to Si29 and Si30, respectively. They were grouped together with the other framework atoms by applying a shared site occupancy with Si29 and Si30. Four oxygen atoms coordinated with these disordered Si atoms were found to be split into two positions each, as suggested by SHELXL during the refinement. The disordered oxygen atoms were divided into two groups, associated with either Si29 and Si30 (assigned as O63â66) or Si31 and Si32 (assigned as O67â70). The PART instruction was used to group the disordered atoms, designated as PART 1 for Si29,30 and O63â66 and PART 2 for Si31,32 and O67â70. The structure including the disordered parts and later with the OSDAs (see below) was refined using restraints on some SiâO bond lengths and bond angles. The occupancy of the two disordered parts was refined to be 56% for PART 1 and 44% for PART 2.
Location of the OSDAs by low-dose cryo-cRED
Although the cRED data collected at room temperature gave high resolution (0.80âà ), it was not possible to locate the OSDAs in the pores. Therefore we collected new cRED data under cryogenic conditions (90âK) using a 40 times lower electron flux density (0.0025âeââà â2âsâ1) to mitigate electron-induced damage to the organic molecules (Supplementary Table 3). The low-dose cRED datasets were first indexed in P2/m using the XDS software to extract integrated intensities. To improve completeness and redundancy, ten datasets were scaled and merged into a single reflection file using XSCALE. Owing to the low dose, the data resolution was reduced to 1.08âà , based on the CC1/2 value. To locate the atomic position of OSDAs, a difference electrostatic potential Fourier map was calculated using the high-resolution framework model of the as-synthesized ZMQ-1 with the positional disorder against the merged low-dose cRED dataset. Two symmetry-independent OSDAs could be located from the difference electrostatic potential map. The positions of the phosphonium cations and carbon atoms in the octamethylene chains could be located and refined using soft restraints. The occupancies of the OSDAs were also refined to be 1.00 for OSDA1 and 0.70(5) for OSDA2. The tricyclohexyl groups could not be located, presumably because of their flexibility.
Catalytic cracking of VGO
The ZMQ-1 samples used for catalytic tests were synthesized in hydroxide medium, USY and Beta were supplied by Zeolyst and UOP, respectively, and MCM-41 was purchased from Tianjin Yunli Chemical Company. The VGO was provided by Sinopec Qingdao Petrochemical Co., Ltd. and its composition and properties are listed in Supplementary Table 7. The catalytic test was carried out in a fixed-bed reactor at 500â°C. The aluminosilicate zeolite catalysts were pelletized, crushed and sieved to 0.18â0.25âmm (60â80 mesh), 0.25â0.38âmm (40â60 mesh) and 0.38â0.83âmm (20â40 mesh) to screen out the appropriate pellet size to mitigate the influence of diffusion limitations. The pellet size range 0.38â0.83âmm was then selected and used for the systematic tests based on the experiment results (Supplementary Tables 8 and 9). In a typical experiment, 1.0âg catalyst (20â40 mesh) was mixed with 4âg quartz sand (20â40 mesh) and loaded into a stainless/quartz reactor tube with a diameter of 20âmm. Before the test, the catalyst was treated at 500â°C in N2 flow at 40âmlâminâ1 for 20âmin; then, the temperature was adjusted to the corresponding reaction temperatures. 1.7âg of VGO was injected at a constant rate on the catalyst bed with nitrogen as the inert carrier gas at a flow rate of 50âmlâminâ1. Gaseous products were collected in a sample bag. The liquid products (C5+) were collected in an ice bath downstream, which was kept at around 0â°C. The spent catalyst was stripped by nitrogen gas for about 30âmin to recover the entrapped hydrocarbons. The gas and liquid products collected after the reaction were analysed using gas chromatography. Gases were analysed using an Agilent 7890A equipped with a HP-PLOT Al2O3 KCl column connected to a flame ionization detector for analysing C1âC6 hydrocarbons and Porapak-Q with a 5A molecular sieve column connected to thermal conductivity detectors for analysing H2, N2, CO, CO2, H2S and O2. Liquids were analysed using a Agilent 7890A equipped with DB-1 columns connected to a flame ionization detector for analysing gasoline, diesel and heavy oil components. After completing the VGO cracking performance test of the catalyst, the spent catalysts were regenerated at 650â°C under flowing air for calculating coke deposition and the regenerated catalysts were also applied for the next catalytic tests. The VGO conversion and product selectivity of dry gas (H2, H2S, CH4, C2H4, C2H6), liquefied petroleum gas (C3,4), gasoline (C5+, boiling point <200â°C) and diesel (C12+, boiling point is 200â365â°C) were defined as follows:
$$\begin{array}{c}{\rm{Conversion}}\,( \% )=({W}_{{\rm{F}}}-{W}_{{\rm{H}}})/{W}_{{\rm{F}}}\times 100 \% \\ {\rm{Dry}}\,{\rm{gas}}\,{\rm{selectivity}}\,({\rm{wt}} \% )={W}_{1}/({W}_{1}+{W}_{2}+{W}_{3}+{W}_{4}+{W}_{{\rm{c}}})\times 100 \% \\ {\rm{Liquefied}}\,{\rm{petroleum}}\,{\rm{gas}}\,{\rm{selectivity}}\,({\rm{wt}} \% )={W}_{2}/({W}_{1}+{W}_{2}+{W}_{3}+{W}_{4}+{W}_{{\rm{c}}})\times 100 \% \\ {\rm{Gasoline}}\,{\rm{selectivity}}\,({\rm{wt}} \% )={W}_{3}/({W}_{1}+{W}_{2}+{W}_{3}+{W}_{4}+{W}_{{\rm{c}}})\times 100 \% \\ {\rm{Diesel}}\,{\rm{selectivity}}\,({\rm{wt}} \% )={W}_{4}/({W}_{1}+{W}_{2}+{W}_{3}+{W}_{4}+{W}_{{\rm{c}}})\times 100 \% \\ {\rm{Light}}\,{\rm{olefin}}\,{\rm{selectivity}}\,({\rm{wt}} \% )={W}_{{\rm{o}}}/({W}_{1}+{W}_{2}+{W}_{3}+{W}_{4}+{W}_{{\rm{c}}})\times 100 \% \end{array}$$
in which WF is mass of feed injected (g), WH is mass of heavy oil with a boiling point above 365â°C in product, which is treated as an unreacted heavy oil, W1 is mass of materials of dry gas components, W2 is mass of materials of liquefied petroleum gas components, W3 is mass of gasoline components, W4 is mass of diesel components, Wc is the mass of coke deposition and Wo is the mass of specific light olefin in all products excluding the heavy oil.
Notably, for each VGO cracking run, a full mass balance was obtained. If the material balance was less than 95% or greater than 105%, the test was repeated. On the basis of the proposed possible reactions and detailed components obtained from gas chromatography, the mass balance is calculated as follows:
$${\rm{Mass}}\,{\rm{balance}}=({W}_{1}+{W}_{2}+{W}_{3}+{W}_{4}+{W}_{{\rm{c}}}+{W}_{{\rm{H}}})/{W}_{{\rm{F}}}\times 100 \% $$
We carried out the tests at different weight hour space velocities (WHSVs; Cat/Oil ratios) for both USY and ZMQ-1(CW), and the data are summarized in Supplementary Fig. 6a and Supplementary Table 10. Five WHSVs were tested, that is, 44, 87, 175, 350 and 874âhâ1 (corresponding to Cat/Oil of 1.18, 0.59, 0.29, 0.15 and 0.06, respectively), by changing the loading amount of zeolites and keeping the feeding amount constant. With the decrease of the WHSV, the VGO conversion increases for both zeolites. For ZMQ-1(CW) and USY, a conversion of 98% was obtained at a WHSV of 87âhâ1, but further decrease of the WHSV to 44âhâ1 resulted in no obvious change in the conversion. Using the WHSV of 87âhâ1, parallel experiments using the fresh ZMQ-1(CW) and USY were performed to eliminate the experimental errors (Supplementary Fig. 7 and Extended Data Table 1). The standard deviation values calculated for the heavy oil conversion rates and product selectivities from three runs are small, proving the high reliability and stability of the reaction system and analysis method we have used in this work. Then, using the WHSV of 87âhâ1, we performed the consecutive reaction and regeneration tests for ZMQ-1(CW) to study the change in activity and deactivation behaviour. The data are shown in Supplementary Fig. 6b and Supplementary Table 11. For the five consecutive runs, no obvious deactivation was detected and the product distribution is almost consistent. The used catalyst was recovered and characterized. It shows that the crystallinity is decreased after reaction but the crystal structure is still retained (Supplementary Fig. 5).
We performed five consecutive reactions without regeneration, which could be considered as quasi-time-on-stream tests, over USY and ZMQ-1(CW) zeolites, to study the deactivation profiles of the catalysts (Supplementary Fig. 8a). Both zeolites show monotonously decreasing activities along with incremental running times, that is, conversion declines from 95% and 96% to 64% and 60% for USY and ZMQ-1(CW), respectively, from the first to fifth run. ZMQ-1(CW) shows slightly lower activity after the initial reaction. Because the coke content could not be analysed using the present method, we further carried out incremental feeding from one to five times for each run, followed by reaction and regeneration, to obtain accumulated coke amount. The coke profiles show that ZMQ-1(CW) has larger accumulated coke, along with incremental feeding and reaction (Supplementary Fig. 8b), over that of USY, indicating that its intrinsic structure might be inducive to the large molecules formation. Comparing with reported optimized USY, Beta and extra-large-pore zeolites at comparable VGO conversion, ZMQ-1 shows higher yield towards fuels, preferentially for diesel, over that of ITT and JZO zeolites (Supplementary Table 12). Moreover, similar propylene yield is detected in ZMQ-1 and ITT.