In April, the US Environmental Protection Agency declared a standard of ‘close to zero’ levels of certain PFAS substances in drinking water.Credit: Justin Sullivan/Getty
A simple chemical bond between carbon and fluorine atoms changed the world — for the better, and then for the worse.
Such bonds lie at the heart of per- and polyfluoroalkyl substances (PFAS), a group of compounds, numbering in the millions, that are remarkably water-, heat- and greaseproof. Discovered in the 1930s with the advent of polytetrafluoroethylene (PTFE, branded as Teflon), these chemicals make pans non-stick and keep the rain off our jackets. Varieties of cosmetics, fire-retardant foam, kitchen utensils, metal coatings, packaging, textiles and more all contain them.
But they have become known as ‘forever chemicals’ because they are difficult to break down, persisting in the environment for perhaps 1,000 years or more. They are also ‘everywhere’ chemicals, in that they can be found in rivers and on the tops of mountains. This would not be such a problem, except that the chemicals are highly toxic, having been linked to developmental problems and conditions ranging from cancer to immune-system suppression.
The world is belatedly starting to act, both to stop forever chemicals entering the environment and to clean up those already there. But more action is needed — and faster.
Catalysts degrade forever chemicals with visible light
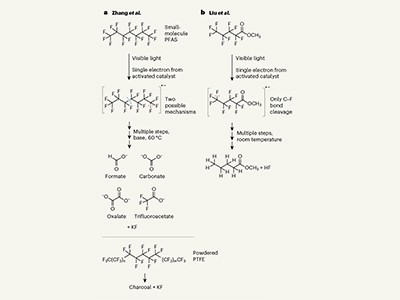
The carbon–fluorine (C–F) bond is one of the strongest in organic chemistry, requiring huge amounts of energy to break down, at huge expense. But now two papers in Nature1,2 describe two low-energy ways to overcome the C–F bond.
Both methods combine a catalyst with some relatively simple chemistry driven by the energy of visible light. In each case, the catalyst absorbs light that then triggers a reaction.
Chemist Garret Miyake at Colorado State University in Fort Collins and his colleagues use this absorbed energy to reduce the C–F bond to carbon–hydrogen — albeit not in Teflon1. Yan-Biao Kang, a chemist at the University of Science and Technology of China in Hefei, and his colleagues uses this energy to break the bond and the overall molecule down to smaller constituent parts2, in temperatures as low as 40 °C. Both papers, without doubt, mark a major step forward.
Important next steps include using these ideas in real-world settings, for example to develop catalysts that work in waste water or that can be used to clean up PFAS in contaminated soils. If a method can be adapted so that it is powered by sunlight, that would be of huge benefit.
Update regulations
Any solutions that emerge from such developments will address only part of the problem. There’s an urgent need both for updated regulation of chemicals, and for more research into safer alternatives to PFAS that do the same things without harming human health and the environment.
On the regulatory front, scientists advising the European Chemicals Agency are considering a proposal from countries including Germany and the Netherlands to ban 10,000 PFAS substances currently in everyday use.
At the global level, the Stockholm Convention, an international agreement that bans persistent organic pollutants, is revisiting its latest list which classifies only three types of PFAS as banned substances.
Could the world go PFAS-free? Proposal to ban ‘forever chemicals’ fuels debate
It is equally important to stop PFAS entering the water supply. In 2021, the European Environment Agency (working under the European Union’s Drinking Water Directive) for the first time set upper limits on PFAS levels in water. In the United States, the Environmental Protection Agency has gone further. In April, it set safe limits for drinking water, under which there will be ‘close to zero’ levels of certain PFAS.
The European proposal doesn’t yet extend to banning PFAS in applications such as medicine or transport, for the simple reason that these chemicals are still too useful and adequate alternatives are yet to be found. C–F bonds in pharmaceuticals allow molecules to remain stable, which is necessary for products’ shelf life.
Medicinal chemists are trying to make drug molecules that contain C–F bonds but can biodegrade safely once they leave the body. In some circumstances, PFAS have replaced other harmful chemicals — for example, outdated, ozone-destroying chlorofluorocarbon (CFC) refrigerants. Non-fluorinated refrigerants are also available, including ammonia and carbon dioxide, but large-scale roll out will need regulatory input.
A lot of clever chemistry, together with updated regulation, will be needed to ensure that forever chemicals finally become never chemicals.