It is common knowledge that the human gut is home to hordes of bacteria, most of which seem essential for good health. But there is another component of the gut microbiome that receives less attention: viruses.
Wherever there are bacteria, viruses called bacteriophages lurk in prodigious quantities. These bacteria-killing microorganisms, first noted in the early twentieth century, are the most abundant biological entities on Earth. An estimated one billion bacteriophages, or phages, reside in one gram of faeces. However, despite their considerable number, the collection of viruses in the gut, known as the virome, is still an unexplored frontier. “The scale of what’s unknown within phages is enormous,” says Martha Clokie at the University of Leicester, UK.
Nature Outlook: The human microbiome
Around three-quarters of the phages that scientists have sequenced infect only 30 bacterial genera1. “Our virome is hugely abundant and incredibly diverse, and we’ve looked at just a tiny percentage,” says Jeremy Barr, a phage biologist at Monash University in Melbourne, Australia.
This dearth of knowledge is not owing to a lack of interest — the US National Institutes of Health has a programme dedicated to cataloguing and characterizing the viruses that live inside the human gut. Instead, the problem is often that the viruses that reside in the gut are difficult to study. But as sequencing and culturing techniques have improved, evidence has begun to build for the idea that phages are key players in the ecology of the gut microbiome, and influence human health for good and for ill.
Virgin territory
Researchers’ knowledge of phages is biased towards those that infect harmful bacteria such as Salmonella species and Escherichia coli, largely owing to interest in using them to fight pathogens — a concept known as phage therapy. Viruses that hunt the harmless commensal gut bacteria that make up the microbiome, by contrast, are much less well catalogued. When microbiologist Colin Hill and his colleagues at University College Cork in Ireland sequenced the guts of 10 individuals in 2019, they turned up about 40,000 viral genetic fragments, only 241 of which were closely related to phages that had been seen before2.
Even huge hauls of viral sequences are likely to be an underestimate of the mind-boggling viral diversity in the gut. Although advances in DNA-sequencing technology have boosted knowledge of double-stranded DNA viruses, the single-stranded DNA and RNA phages often slip through the net owing to equipment availability and cost.

Colin Hill thinks scientists need to return to bacterial cultivation to discover more phages.Credit: Colin Hill
Many phages are thought to infect just a few or even one strain of bacteria. But it is often difficult to determine what a particular phage does from its genetics alone. “A lot of the time, we cannot even tell you the host it infects,” says Barr.
In a 2014 study, software for piecing together DNA fragments revealed a family of viruses that the authors of the study called crAssphages, which are extremely abundant in the human gut3. Some of these had genomes of a size akin to bacteria; in some people, these phages account for 90% of all viral DNA in the gut. But when the crAssphage genes were cross-checked with known microbes, there were few matches. “This organism is in such high abundance in all our guts, and yet we have no idea what 40–50% of their genes do,” says Scott Handley, a microbiologist at Washington University in St. Louis, Missouri. “We call this functional dark matter.”
Techniques such as identifying DNA sequences that bacteria have captured from phages and inserted into their own DNA can shed some light on which phages infect which hosts. (This is a primitive form of immune memory for the bacteria, and is the basis of the CRISPR–Cas9 genome-editing technique.) An approach from biotechnology firm Phase Genomics in Seattle, Washington, is also allowing scientists to catch phage DNA in the act of infecting bacteria cells. The firm uses chemical crosslinking to freeze phage DNA that is in contact with bacterial DNA.
However, for almost five years after they were discovered, crAssphages had still been studied only on computers, says Hill. In his view, reliance on sequencing has become a double-edged sword. “We learnt more about the types of phages present, but paradoxically we moved away from growing them on their hosts and understanding their biology,” he says.
Many phage biologists, including Hill, are convinced that it is time to return to Petri dishes and fermenters, and to cultivate more gut bacteria to reveal phages. “We know more and more about the genomes of phages, and less and less about how they actually behave,” he says.
Culture wars
When Clokie opened her lab in Leicester in 2007, she noticed that there was surprisingly little research into phages that target Clostridium difficile, a bacterium that can cause stomach cramps, diarrhoea and even lethal damage of the lower bowel. “In the naivety of youth and blind optimism, I thought this is an interesting pathogen and I don’t see why people haven’t found phages,” recalls Clokie. Years later, having finally determined the activity of a handful of phages for C. difficile, she understood why others had made such little progress4.
First, the bacterium itself was challenging to grow in the lab. “Five years ago, the predominant view was that most gut bacteria were unculturable,” says Barr. This is no longer considered to be true. “Things have changed. We can now grow the majority,” he says. Gut bacteria can be grown in oxygen-free environments and on specialized media that provide the right nutrients for many of these microbes.

Martha Clokie (left) looks for bacteriophages in a bacterial ‘lawn’.Credit: University of Leicester
But growing the bacteria is only the first step in culturing the phages that prey on them, and just one of the difficulties that Clokie and other researchers face. Some strains of bacteria bristle with anti-viral armaments, preventing the phages from establishing a presence. And even when the host strain is suitable for phages to replicate, it can then be difficult to spot them.
A classic biology experiment involves growing E. coli on plates and infecting them with T4, a bacteriophage with the appearance of a tiny six-legged robot, which was first described in the 1940s. The phage hijacks the bacterium and forces it to produce copies of the phage, then break apart and release the progeny. The destruction of bacterial colonies can be seen with the naked eye. Unfortunately, the same cannot be said for most phages that infect gut bacteria. “Many phages do not form plaques or zones of clearing and are difficult to detect or count,” Hill explains.
Researchers are getting better at overcoming these problems and culturing phages, but it remains an involved and time-consuming process. This poses a problem for funding: it is difficult to apply for money to study a virus that you don’t already have. But obtaining it takes time, energy and money. It might be possible to persuade a student to take on the challenge, Clokie says, but for them “it is high risk; they might not get anything for their PhD”. With as many as ten million species of phage to be studied, “the task is monumental”, Hill adds.
Let’s dance
When researchers have succeeded in culturing phages, their observations have at times defied expectations. In 2018, Hill finally reported on the propagation of crAssphages in Bacteroides intestinalis5. This led to a structural description of the virus particle using an electron microscope6. But just as importantly, it allowed the team to scrutinize how the phage interacted with its prey.
Given phages’ reputation as bacterial mass murderers, Hill expected to witness a duel to the death. To his surprise, crAssphages and bacteria instead settled into a comfortable relationship, growing side by side. “The bacteria seem to have evolved to support the presence of the phage,” says Hill. “They’re not actually fighting. They’re dancing.”
Phages might therefore be better thought of not as bacterial assassins but rather as levers in the complex ecosystem that is the human gut. Many phage biologists reach for zoological analogies, such as the big cats of Africa’s savannahs that pick off diseased and unhealthy animals. “Lions keep the herds of antelope fit,” says Hill — the same could be true of phages and bacteria. “There’s a symbiotic relationship,” agrees Barr. “Your virome gives your microbiome more flexibility and capacity to evolve.”

Phage biologist Jeremy Barr says there is a symbiotic relationship between phages and bacteria.Credit: Jeremy Barr
In some instances, this is bad news for human health. The Shiga toxin that can trigger diarrhoea in humans, for example, is encoded into some E. coli strains by a phage. One suggestion is that this defends bacteria against a protozoan predator — beneficial to the phage and its host, but with unfortunate side effects for people. Predation by phages also encourages bacteria to change their outer protein shell — an attempt to evade the phage, but also a move that can help bacteria to escape the attention of the human immune system. “Phages keep bacteria nimble and fit and heterogeneous, keeping them changing their coats, changing their structures, switching on and off receptors,” says Hill.
In other ways, however, the complex dance between phages and bacteria in the gut might benefit human health. Low diversity of microbes in the gut has been linked to a variety of conditions, including inflammatory bowel disease (IBD), C. difficile infection, liver disease and diabetes. A robust population of phages could help to keep diversity high by evolving to target the most rapidly proliferating bacterial strains, many of which are pathogens. “Phages adapt to kill common bacteria,” says Britt Koskella, an evolutionary biologist at the University of California, Berkeley. “In doing so, they make space for more rare bacteria and therefore increase microbiome diversity.”
Disease links
As researchers’ understanding of the phages in the human gut improves, their influence on health is becoming clearer. Sequencing studies have revealed differences in the phages present in healthy people and those with disease. “Ranging from infectious diseases to metabolic syndromes, you see clear viral signatures of microbiome disturbances,” says Koskella.
Handley thinks that phages might have a role in triggering IBD, a condition that can cause diarrhoea, abdominal pain and weight loss. While he was hunting a human infectious virus that might explain the condition, he instead found a drastic spike in the number of phages present in people with IBD compared with those present in healthy individuals7.
Faecal transplants can treat some cancers — but probably won’t ever be widely used
He thinks that inflammation might stress bacteria and cause phages hidden in them to jump from what they see as a sinking ship. This would boost the number of free phages in the gut. “Once you get inflammation, the bacteria become stressed and that releases the phage,” says Handley. “Those phages then start reshaping the bacterial communities.” The phages might also provoke further immune activity. Handley and his colleagues reported that viromes from people with IBD triggered a pro-inflammatory response on macrophages — a type of immune cell that kills microbes8. The logical conclusion, he says, is that these phages turn up the immune dial and perpetuate inflammation in the gut.
Handley proposes that a spike in certain phages might offer a warning flag for the inflammatory flare-ups that people with IBD experience. He also thinks that phage populations might contribute to failures of faecal microbiota transplants in people with IBD, in which faecal matter is transferred from healthy donors to restore a healthy microbiome. “It could be some of these therapies aren’t as successful as people would like them to be because the phages are killing off the probiotics,” he says.
Phages as therapies
Even as potential links with disease are being uncovered, researchers are exploring how phages might be put to good use.
Hill proposes that probiotic bacteria could be cultured with their phages. At first glance, this seems likely to be unpopular with probiotic producers — phages are notorious among cheesemakers, for example, for eliminating the cultures of bacteria that are added to milk to make cheese, and Hill admits that phages could similarly wipe out some vats of probiotic cultures if producers were to deliberately add them to the mix. The benefit, however, is that the microbes that did survive would be fitter, and perhaps more able to compete with other bacteria and resist phages when introduced into the wilds of a person’s intestinal tract.
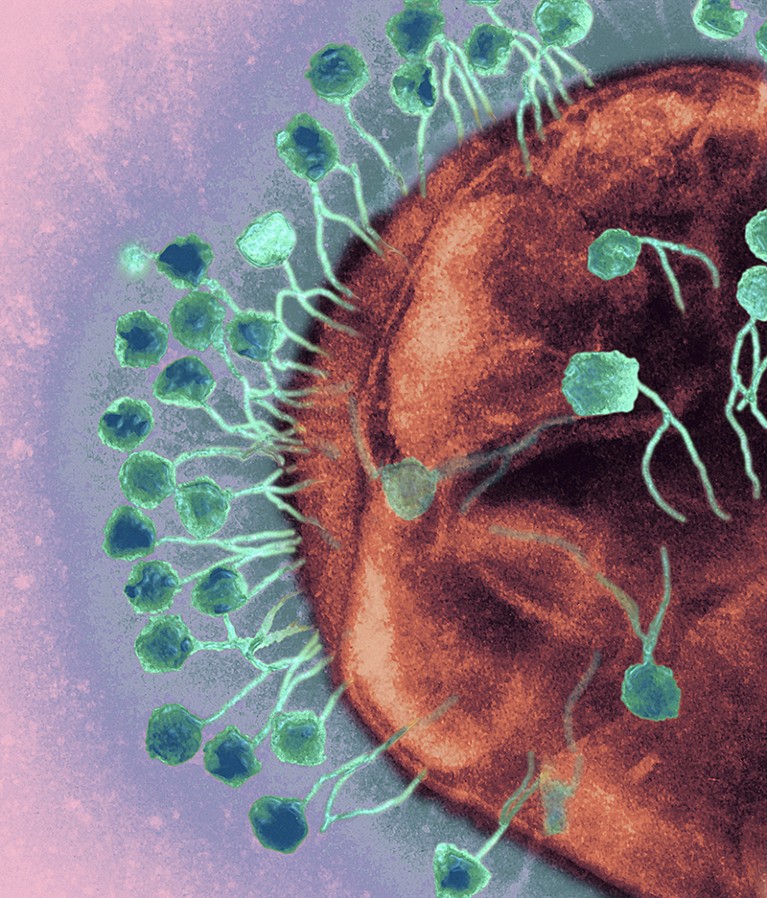
A transmission electron micrograph shows bacteriophages (turquoise) attacking a bacterial cell (red). Credit: Ami Images/Science Photo Library
Efforts are also under way to use phages themselves as therapies. Faecal transplants are an established, effective therapy for people with C. difficile infection. These treatments are usually thought of as transplants of bacteria, but phages are inevitably part of the brew. “Even two years after faecal microbiome transplants, you can still detect phages from the donor,” says Hill. In 2017, a study provided evidence that filtering the bacteria out of the material to be transplanted, and delivering only phages and the molecular milieu, has a similar effect to the full faecal-matter transplant in people with C. difficile infections9. “We are going to see more subtle uses of phages to modulate microbiomes to a favourable state,” says Clokie.
Last year, a group in China reported that administering a cocktail of phages in a mouse model of diabetes suppressed opportunistic microbes, while supporting more beneficial bacteria10. And in February, researchers reported that transferring phages from healthy mice into those with chronic stress prevented expected shifts in their gut bacteria and in their behaviour11. This suggests that phages might even be used to rebalance gut microbes that influence brain function.
Turning these faint possibilities into effective, useable therapies is a big job, and one that is not likely to be completed any time soon — not least because of the continuing difficulty of working with phages in the lab. But many researchers are determined to persevere. “When I’ve got phages, not just sequences, I can produce them, test them and apply them to an animal model,” says Barr. “That all gives a lot more biological understanding.”
It also brings the vast menagerie of viruses that co-evolved with our gut bacteria closer to being testable in people as treatments. “Including phages is a trick we are missing in microbiome science. We should consider using these viruses with bacteria,” says Hill.




