Cell counting
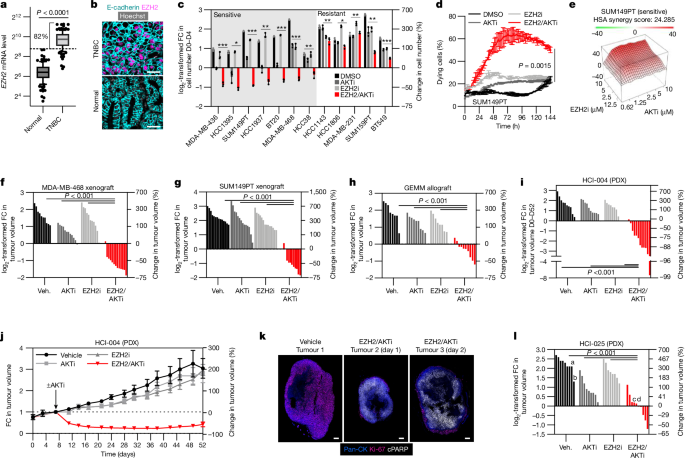
To measure cellular proliferation and cytotoxicity, manual cell counting experiments were conducted. On day â6, cells were seeded at 40% confluency in 10âcm dishes. On day â5, cells were treated with DMSO or tazemetostat. On day â3, cells were passaged 1:2 and maintained in tazemetostat or DMSO. On day â1, cells were seeded at 250,000 cells per well into six-well tissue culture dishes for counting (enough for day 0 and for day 4 counts) and 625,000 cells per 6âcm dish for protein lysate collection. For cell counting assays including siRNA, cells were transfected on day â1 and incubated with siRNA for 6â8âh before seeding. On day 0, starting cell counts were measured using a haemocytometer and the remaining cells were dosed with DMSO, ipatasertib, tazemetostat or the combination. On day 1, protein lysates were collected. On day 4, remaining cells were counted using a haemocytometer. The log2-transformed fold change in cell number was calculated by normalizing day 4 cell count values to day 0 cell count values. For days â6 to â1, cells were cultured in appropriate medium containing 10% FBS. For days 0 to 4, cells were cultured in appropriate medium containing 2% FBS. All of the experiments were completed at least three times. Statistical methods were not used to pre-determine sample size. Blinding was not conducted. Cells were seeded from a master solution and allocated across all compared conditions to control for seeding and population differences.Â
IncuCyte live-cell imaging
Live-cell imaging was completed using IncuCyte live-cell imaging. After 5âdays of pretreatment with tazemetostat or DMSO, cells were seeded at 5,000 cells per well in 96-well plates. Then, 24âh after seeding, cells were treated with tazemetostat and/or ipatasertib in medium containing NucLight Rapid Red Reagent (Sartorius, 4717) to label nuclei and Cytotox Green (Sartorius, 4633) to label cytotoxic cells. Plates were placed inside the IncuCyte machine and images were acquired every 2âh for 5âdays. The percentage of cytotoxic cells was determined using the integrated IncuCyte software by quantifying the overlap of NucLight- and Cytotox-green-positive cells divided by the total number of NucLight-positive cells. Four images per well were taken and each condition was seeded in duplicate. All of the experiments were completed at least three times.
Synergy score analysis
To measure synergistic interactions between EZH2 inhibitor tazemetostat combined with PI3KâAKT pathway inhibitors, cells were seeded in 96-well white flat bottom plates at 5,000 cells per well. For each condition, two technical replicates were seeded per combination treatment. After 5âdays of pretreatment with EZH2 inhibitor, cells were dosed with PI3KâAKT pathways inhibitors. Drugs were added at the following concentrations: EZH2 inhibitor tazemetostat (0, 1.25, 2.5, 5, 10âμM), and AKT inhibitor Ipatasertib (0, 0.625, 1.25, 2.5, 5, 10âμM). After 96âh of treatment, cell viability was quantified using CellTiter-Glo (Promega, G9291) and normalized to DMSO to calculate the inhibitory response. SynergyFinder was then used to calculate the synergy score using the Gaddumâs non-interaction modelâhighest single agent (HSA) model, where a value of greater than 10 indicates a synergistic interaction56.
Orthotopic xenografts
All mouse work was done in compliance with the Institutional Animal Care and Use Committee (IACUC) at Brigham and Womenâs Hospital. The maximum tumour size was 20âmm in one dimension of the tumour. SUM149PT and MDA-MB-468 xenografts were generated by injecting 3âÃâ106 cells using a 1:1 ratio of DMEM/F12 and Matrigel (Corning, 356231) into the fourth inguinal mammary fat pad of nude mice. For the organoid allograft, organoids were generated from Trp53fl/fl mice and cultured as previously described23,24 and 50,000 cells were implanted into the fourth inguinal mammary fat pad of nude mice. For SUM149PT and MDA-MB-468 xenograft studies, two tumours per mouse were implanted. For the organoid allograft, MDA-MB-468 xenograft survival study and PDXs, one tumour per mouse was implanted. Tumour fragments of HCI-025 and HCI-004 were implanted into NSG mice. Tumours arose after 2âweeks and were enrolled when they reached 100â250âmm3. The tumour volume was measured using vernier callipers with the formula (widthâÃâwidthâÃâlengthâÃâ0.52). For the treatment schedule, mice were pretreated with tazemetostat or vehicle for 7 days before being treated with vehicle (ipatasertib vehicle + tazemetostat vehicle), ipatasertib (ipatasertib + tazemetostat vehicle), tazemetostat (ipatasertib vehicle + tazemetostat) or combination (tazemetostat + ipatasertib). Ipatasertib was dosed at 50âmg per kg once per day through oral gavage. Ipatasertib was prepared in 0.5% methylcellulose, viscosity 4,000âcP, with 2% Tween-80 at pHâ7.0 and kept at 4â°C for up to 1âweek. Tazemetostat was dosed at 250âmg per kg twice a day through oral gavage. Tazemetostat was prepared in 0.5% methylcellulose containing 0.1% Tween-80 and kept at 4â°C for up to 4 weeks. PDXs HCI-025 and HCI-004 were prepared and passaged as previously described25,57. Sample size was pre-determined: we calculated that if we used 10 mice per treatment group, we would have 87% power to detect a 50% reduction in tumor size, and every effort was made to reach that sample size. Animals were randomized into treatment arms. Blinding was not conducted. The temperature in rooms housing mice was kept stably between 68 and 75â°C. The humidity range of rooms housing mice was kept stably at 35â65%, with 50% humidity being considered optimal. Animals experienced 12âh of light during the day and 12âh of dark during the night with a light intensity between 100â250 lux. Mice were offered diet ab libitum in food hoppers hung from the top of the cage with Pico 5052 Irradiated Rodent Chow or Pico 5058 Irradiated Mouse Chow. Mouse were offered water ad libitum. Mice were housed in Allentown ventilated cages with bedding approximately 1/2 inch in depth with Alpha-blend or crinkled paper (nude mice received paper-based bedding only). Mice were offered gnawing enrichment in the form of nestlets or popsicle sticks (nude mice were offered only popsicle sticks). Raw animal data are provided in Supplementary Data 2.
Cell lines
Cell lines were purchased directly from ATCC or authenticated using STR analysis (Labcorp). SUM149PT and SUM159PT cells were cultured in DMEM/F12 (Gibco, 11330-057), 293T, RPE1, MDA-MB-157, MDA-MB-231, MDA-MB-436 and MDA-MB-468 cells were cultured in DMEM (Corning, 10-013-CV), and BT20, BT549, DU4457, HCC38, HCC70, HCC1143, HCC1187, HCC1395, HCC1806, HCC1937 and HCC2157 cells were cultured in RPMI (Corning, 10-040-CV), IMR90 and BJ fibroblast cell lines were cultured in EMEM (Thermo Fisher Scientific, 10-009-CV). All media were supplemented with 10% FBS, and 1Ã concentration of penicillinâstreptomycinâglutamine (Gibco, 10378016). TP53â/â organoids were generated and cultured as previously reported23,24. MCF10A cells were cultured as previously reported58. No commonly misidentified cell lines were used in this study. Cell lines were routinely found to have no mycoplasma contamination using Lonza MycoAlert PLUS Mycoplasma Detection Kit.
Crystal violet
Cells were seeded at 50,000 cells per well into 12-well plates and then stained with crystal violet at the indicated times. In brief, after the medium was removed, wells were washed with PBS three times before fixation with 2% formaldehyde (1:2 dilution of Formalde-Fresh Solution; SF94-4) for 15âmin a room temperature. After fixation, wells were washed with PBS once before staining with 0.025% crystal violet (Sigma-Aldrich, C6158) for 2âh. After staining, wells were washed with water four times before air drying and imaging using a standard scanner.
Immunohistochemistry
Tumours were collected for immunohistochemistry 4âh after final dose of drug treatment by removing tumour and fixing in Formaldehyde-Fresh (Thermo Fisher Scientific, SF94-4) for 24âh. After 24âh, tumours were stored in 70% ethanol before sectioning and analysis. For haematoxylin and eosin staining, sectioning and staining was performed at the Harvard Medical School Rodent Histopathology Core. For Ki-67 and EZH2 (BD Bio, 612667) immunohistochemistry, sectioning and staining was performed at the Brigham & Womenâs Hospital Pathology Core.
siRNA and CRISPR
Cells were incubated for 6â8âh with 0.1âµM siRNA constructs using a 1:400 dilution of Lipofectamine RNAiMAX Transfection Reagent (Invitrogen, 13778075) in antibiotic-free medium. siRNA ON-TARGETplus pools were purchased from Horizon Biosciences: control (D-001810-10-50), GATA3 (L-003781-00-0005), JAK1 (L-004393-00-0010), BMF (L-003145-00-0005), STAT3 (L-003544-00-0005), IL-6R (007994-00-0005), BACH2 (L-009787-00), HSF1 (L-012109-02), MEF2D (L-009884-00), NFIA (L-008661-00), NFIX (L-009250-00), PRDM1 (L-009322-00), RELB (L-004767-00), ZNF143 (L-013965-00), ZNF354C (L-014199-02), BACH1 (L-007750-00), ELF3 (L-016080-00-0005), FOS (L-003265-00), FOXO1 (003006-00-0005), FOXO3 (003007-00-0005), HOXC11 (L-017602-00-0005), HOXD11 (L-013095-00-0005), HOXD12 (L-013096-02-0005), JUN (L-003268-00), MAFK (L-008580-00), SIX2 (L-017024-00), ETV1 (L-003801-00), ETV4 (L-004207-00), FOSL1 (L-004341-00), FOSL2 (L-004110-00), HOXC10 (L-017601-00), JDP2 (L-008321-01), JUNB (L-003269-00), JUND (L-003900-00), MAFF (L-003903-00), MAFG (L-009109-00), MAFK (L-008580-00), MITF (L-008674-00), MXI1 (L-009947-00), PBX3 (L-020121-00), PKNOX1 (L-021413-00), SIX1 (L-020093-00), SREBF2 (L-009549-00), TBX1 (L-012195-00), USF1 (L-003617-00), USF2 (L-003618-00) and VDR (L-003448-00). shRNA constructs in the pLKO.1 backbone against non-targeting and GATA3 (TRCN0000019299) were purchased from Sigma-Aldrich. sgRNA constructs in the pLentiCRISPR-v2 vector expressing Cas9 against control, STING and GATA3 were purchased from GenScript.
Inhibitors
For all assays except synergy assays, tazemetosat was used at 5âμM (S7128, Selleckchem), ipatasertib was used at 5âμM (S2808, Selleckchem), BYL719 was used at 5âμM (S2814, Selleckchem), GDC0077 was used at 5âμM (S8668, Selleckchem), GDC0941 was used at 3âμM (S1065, Selleckchem), MAK683 was used at 5âμM (S8983, Selleckchem) and itacitinib (JAK1i) was used at 500ânM (S7812, Selleckchem). TBK1 inhibitor GSK8612 was used at 16âμM (S8872, Selleckchem). Anti-IL-6R antibody is tocilizumab and was supplemented at 50âμgâmlâ1 (A2012-5MG, Selleckchem). STING agonist ADU-S100 (MIW815) was supplemented at 50âμM (CT-ADUS100, Chemietek). Docetaxel was supplemented at 2ânM or 10ânM (S1148-10MM/1ML, Selleckchem).
ELISA
Human IL-6 (Thermo Fisher Scientific, KHC0061) and 2â²3â²-cGAMP (Cayman Chemical, 501700) were detected with ELISAs according to the manufacturerâs instructions. Samples for IL-6 (conditioned media) or 2â²3â²-cGAMP (protein lysates) were collected 24âh after addition of AKTi and/or TBK1i. Values represent the average of three or four biological replicates normalized to the DMSO sample.
GATA3 expression plasmid
GATA3 ORF was purchased from Addgene (plasmid 81902) in pDONR221 vector59 and cloned into pHAGE-puro expression vector (Addgene plasmid 118692) using LR clonase II (11791020; Thermo Fisher Scientific) according to the manufacturerâs specifications. GATA3 overexpression was enforced in SUM159PT cells by lentiviral transduction and puromycin selection (1âμgâmlâ1) for 72âh.
Western blotting
Protein was collected after 24âh of vehicle, single agent or combination treatment. Cells were washed with PBS (806544-500ML, Sigma-Aldrich) and then boiling 1% SDS lysis buffer (1% SDS (15553-035, Invitrogen), 10âmM Tris-HCl pHâ7.5 (77-86-1, Sigma-Aldrich), 100âmM NaCl (S5586, Sigma-Aldrich)) was directly added to plates. Plates were scraped and protein lysate was sheared using 20âG needle 5â6 times. Proteins were boiled at 95â°C for 10âmin and centrifuged at maximum speed for 3âmin. The protein concentration was determined using the BCA quantification method (23222, Bio-Rad). Proteins were run in SDSâPAGE gels (4561084, Bio-Rad) and transferred to Immobilon PVDF membranes (IPVH00010, Sigma-Aldrich). Membranes were blocked with 5% milk in TBST for 1âh before incubation with primary antibodies overnight. The membranes were incubated with HRP-conjugated secondary antibody corresponding to the species of the primary antibody. HRP signal was measured using autoradiography film or by BioRad Chemidoc. Antibodies for western blotting were as follows: H3K27me3 (9733S, Cell Signaling Technologies, 1:1,000), H3 (4499S, Cell Signaling Technologies, 1:1,000), PRAS40 (2610S, Cell Signaling Technologies, 1:1,000), pPRAS40 (2997S, Cell Signaling Technologies, 1:1,000), GAPDH (2118S, Cell Signaling Technologies, 1:1,000), GATA3 (558686, BD BioSciences, 1:250), pSTAT3 (sc-8059, Santa Cruz, 1:500), STAT3 (9139, Cell Signaling Technologies, 1:1,000), JAK1 (3332S, Cell Signaling Technologies, 1:1,000), P27 (sc-528, Santa Cruz, 1:1,000), FOXO1 (9454, Cell Signaling Technologies, 1:1,000), FOXO1-pS265 (9461, Cell Signaling Technologies, 1:500), TBK1 (3504S, Cell Signaling Technologies, 1:1,000), TBK1-pS172 (5483S, Cell Signaling Technologies, 1:1,000), STING (13647S, Cell Signaling Technologies, 1:1,000), anti-rabbit secondary (111-035-144, Jackson ImmunoResearch, 1:5,000), anti-mouse secondary (115-035-166, Jackson ImmunoResearch, 1:5,000). All uncropped images of immunoblots are provided in Supplementary Data 1.
STINGâTBK1 co-IP
Cells were treated with the indicated drugs and then collected after 8âh of treatment by washing with PBS and scraping. Pelleted cells were lysed using EBC lysis buffer (50âmM Tris, pHâ8.0, 150âmM NaCl, 0.5% NP-40, 1:10,000 β mercaptoethanol, 0.5âmM EDTA) supplemented with protease inhibitors (Sigma-Aldrich, 11836153001) and phosphatase inhibitors (Sigma-Aldrich, 4906837001). Cell lysates were incubated on ice for 20âmin and then cleared by centrifugation at 16,000g for 20âmin. Protein extracts were quantified using BCA reagent and then equal quantities were incubated with STING (Cell Signaling, 13647S) or IgG (Cell Signaling, 2729S) antibody and magnetic protein A/G beads (Pierce) overnight with end-over-end agitation at 4â°C. Beads were washed five times with EBC lysis buffer before immunoprecipitated proteins were eluted using SDS sample buffer and analysed by western blotting (described above).
RNA-seq
RNA was isolated after 8 or 24âh of treatment using the RNeasy Plus kit (74134, Qiagen). RNA was sequenced at the Dana-Farber Cancer Institute Molecular Biology Core Facility (SUM149PT, MDA-MB-468) or Novogene (MDA-MB-468 docetaxel experiment, HCC38, HCC1395, HCC1937) using the Illumina NextSeq 500 system. Raw data were mapped to the Hg19 (SUM149PT, MDA-MB-468) or hg38 (MDA-MB-468 docetaxel experiment, HCC38, HCC1395, HCC1937) genome using STAR and count files were made using HTSeq60,61. DESeq2 was used to normalize counts (mean-ratio method), calculate total reads and determine differentially expressed genes62. Volcano plots were generated using EnhancedVolcano and heat maps were generated using pheatmap63,64. DESeq2 was used to determine differentially expressed genes for public RNA-seq raw counts collected from CCLE database. Volcano plots were generated using the ggplot2 package in R and heat maps were generated using ComplexHeatmap package in R.
ATACâseq
ATACâseq data were sequenced using the Illumina NovaSeq 6000 system. Using the ENCODE pipeline (https://www.encodeproject.org/atac-seq/), raw sequencing FASTQ data were read and aligned to the hg38 reference genome. MACS2 was used to identify peaks indicating open chromatin region. We filtered out non-reproducible peaks by selecting peaks that were detected in replicates of each condition for each cell line, then peaks were merged for overlapping region across all conditions and samples using the GenomicRanges package in R. The consensus peaks were annotated using ChIPseeker package in R. Using featureCounts in Rsubread package in R, we counted all reads over the consensus peak regions, then performed differential accessible peak analysis using DESeq2. Motif discovery and analysis was performed using RGT-HINT. Transcription-factor-binding sites were identified with JASPAR motifs. Transcription factor activity scores were estimated based on the transcription factor footprint profiles. We ranked genes based on chromatin accessibility differences in promotor. If multiple peaks were annotated to single gene, we estimate the major changes by taking maximum/minimum log-transformed fold changes if <80% of peaks are more open/closed. The ranked genes were used for GSEA using the fgsea package in R. Gene sets were collected from the entire MSigDB using the msigdbr package in R.
Machine learning model
For the initial training set, TPMs of RNA-seq data for 17 cell lines (10 sensitive, 7 resistant) were collected from the CCLE dataset and standardized using z-score normalization. Feature selection is important for machine learning training as too many irrelevant features can interrupt model training (for example, overfitting). We therefore selected subset of genes by ranking them in terms of its variance or log-transformed fold change. First, we selected genes that were differently expressed between sensitive and resistant cell lines with FDRâ<0.05. We then ranked the genes based on their variance and selected the top 50, 100 and 200 highly variable genes (represented as top 50, top 100, and top 200, respectively). We also selected genes based on a cut-offs for the log-transformed fold change in sensitive versus resistant of 2 and 5, that is, |LFC|â>â2 and |LFC|â>â5. The models were trained using two different algorithms, RF and SVM, on the training sets with the subset of genes, then evaluated by its accuracy. We validated the models using leave-one-out cross validation. During the validation, the dataset is divided into N subsets where N is the total number of cell lines. Each subset includes Nâââ1 cell lines by removing one cell line as the validation set. For each subset, we performed differential expression analysis, ranked differentially expressed genes, defined a subset of genes based on its rank, trained machine learning models on the subset then tested the models using the validation cell line that was not used in the entire process at all. This was repeated N times by removing one cell line each time. In total, we have 2âÃâ5âÃâN models as we use two algorithms (RF, SVM) and five gene sets (top 50, top 100, top 200, |LFC|â>â2 and |LFC|â>â5). Cross validation accuracy is calculated by the number of correctly predicted unused cell line/N. On the basis of the validation and test accuracy, we selected the best model (RF, |LFCâ|â> 5), then retrained the model using 17 cell lines. We used the final model to predict the sensitivity of TNBC tumours in the TCGA database. Similarly, TPMs of RNA-seq data were obtained then scaled using z-score normalization. Feature selection, including differential expressions and rank genes, were implemented in R. All of the machine learning methods, including training, validation, and testing, were implemented using the scikit-learn library in Python.
Copy-number analysis
To measure copy number alterations in TP53â/â organoid allograft tumours, we completed ultra-low-pass whole-genome sequencing with the Dana-Farber Cancer Institute Molecular Biology Core Facility. Genomic DNA was isolated from a tumour from an untreated tumour-bearing mouse, fragmented and sequenced. Approximately 5âmillion library reads were analysed for copy variation at 100âkb resolution. We used CNVkit pipeline to process the germline sequencing raw data. The scatter plot was generated using the CNVkit library in Python.
ssGSEA and GSEA
ssGSEA was performed using GenePattern (https://www.genepattern.org/)65,66. GSEA was performed using software available online (http://www.gsea-msigdb.org/)65,67. Gene sets are published on the MSigDB for hallmark apoptosis (M5902, HALLMARK_APOPTOSIS)68, mammary stem cells (M2573, LIM_MAMMARY_STEM_CELL_UP)26 and mature luminal mammary cells (M2578: LIM_MAMMARY_LUMINAL_MATURE_UP)26. Mammary involution gene set was derived from a previous study that identified genes upregulated in involution that were dependent on JAK1 expression in the mammary gland37.
TNBCtype analysis
Molecular subtyping of samples using RNA-seq values was performed using the TNBCtype tool27,69. Correlation coefficient with most significantly altered subtypes was reported for each cell line.
BioRender
The model presented in Fig. 6i was created using BioRender (license number IY273VQW9J).
ChIPâqPCR
MDA-MB-468 cells were plated at 1.2âÃâ106 in 15âcm plates and treated the same as for cell counting and immunoblotting. After 24âh of vehicle, single agent or combination treatment, cells were collected in native medium. Cells were cross-linked first with 2âmM DSG (20593, Pierce) for 45âmin at room temperature followed by 1% formaldehyde for 10âmin at 37â°C before quenching with 0.125âM glycine for 5âmin. Chromatin was isolated by first incubating with buffer 1 (50âmM HEPES-KOH pHâ7.5 (15630-080, Thermo Fisher Scientific), 140âmM NaCl (S5586, Sigma-Aldrich), 1âmM EDTA pHâ8 (15575-038, Invitrogen), 10% glycerol (G5516, Sigma-Aldrich), 0.0033% NP-40 (493015, Calbiochem) and 0.25% Triton X-100 (T8787, Sigma-Aldrich)), then resuspending the pellet in buffer 2 (10âmM Tris-HCl pHâ8 (15567-025, Invitrogen), 200âmM NaCl, 1âmM EDTA pHâ8 and 0.5âmM EGTA pHâ8), and finally resuspending the cell pellet with buffer 3 (10âmM Tris-HCl pHâ8, 100âmM NaCl, 1âmM EDTA pHâ8, 0.5âmM EGTA pHâ8, 0.1% sodium deoxycolate (D6750, Sigma-Aldrich) and 0.5% N-laurosarcosine (L7414, Sigma-Aldrich)). Isolated chromatin was then sonicated for 30 cycles of 30âs on, 30âs off using a Diagenode BioRuptor Plus sonicator on âhighâ. Soluble chromatin was immunoprecipitated with FOXO1 (2880, Cell Signaling Technologies), IgG (2729S, Cell Signaling Technologies) or STAT3 (9139, Cell Signaling Technologies) antibodies overnight. Immunoprecipitated chromatin was captured with magnetic protein A/G beads (88803, Pierce). Immunoprecipitated chromatin was washed with low-salt buffer three times (0.1% SDS, (15553-035, Invitrogen), 1% Triton X-100, 2âmM EDTA pHâ8, 20âmM Tris-HCl pHâ8 and 150âmM NaCl), high-salt buffer three times (0.1% SDS, 1% Triton X-100, 2âmM EDTA pHâ8, 20âmM Tris-HC-l pHâ8, 500âmM NaCl), LiCl buffer three times (0.25âM LiCl (62476, Sigma-Aldrich), 1% NP-40, 1âmM EDTA pHâ8, 10âmM Tris-HCl pHâ8, 1% sodium deoxycholate) and TE buffer (10âmM Tris-HCl pHâ8, 10âmM EDTA pHâ8) before elution in elution buffer (50âmM Tris-HCl pHâ8, 10âmM EDTA, 1% SDS). Eluted chromatin was purified using the QIAquick PCR purification kit (28106, Qiagen). Relative abundances of BMF and GATA3 promoters in immunoprecipitated chromatin was measured using qPCR using PerfeCTa SYBR Green SuperMix Reaction Mixes (95054-500, QuantaBio) and primers listed below. The percentage input values were calculated for each immunoprecipitation. Fold change enrichment values were calculated by normalizing to the percentage input of the appropriate IgG control for each immunoprecipitation.
HA knock-in BMF
An N-terminal HA-tag was introduced into the endogenous locus of BMF as previously described70. The crRNA (5â²-TTGCCCCCTCACAGGAGAGA-3â²) was hybridized with Alt-R CRISPR-Cas9 tracRNA (IDT, 1073190) at 95â°C for 5âmin, at an equimolar ratio (0.375ânmol). The mix was cooled down to room temperature on the benchtop for 10âmin before adding 2âµl of Alt-R S.p. Cas9 Nuclease V3 (IDT, 1081059) and 5âµl of 100âµM single-stranded donor oligonucleotide (5â²-GCTGAGGGGGCAGTCCAGTAGGCTCTGGGCAAACAGGTCAGCAGAGAGCAAGCTCCCGGGTTGGGTCACCGGCTCCCCATCCTCTGGTTGGAACACATCATCCTCCAGCTCCTCCACACACTGAGATGGCTCAGCGTAATCTGGTACGTCGTATGGGTACATCTCTCCTGTGAGGGGGCAACGCAGGCATCTGGGCTGCT-3â²). The mix was incubated for 20âmin at room temperature and added to 1.5âmillion (MDA-MB-468) or 1 million (HCC1395) cells resuspended in 100âµl of SF Cell Line Nucleofector solution (Lonza, V4XC-2012). Cells were then transferred to a cuvette and nucleofected using the EO-117 program in a 4D-Nucleofector X Unit (Lonza).
CUT&RUN
CUT& RUN was performed as previously described71. In brief, 500,000 nuclei from MDA-MB-468 cells treated with DMSO, 5âμM ipatasertib, 5âμM tazemetostat or a combination of both drugs were isolated using nuclear extraction buffer (20âmM HEPES pHâ7.9, 10âmM KCl, 0.1% Triton X-100, 20% glycerol and 1âmM MnCl2). Nucleus samples were then immobilized to Biomag Plus Concanavalin A (Con A)-coated magnetic beads (Bangs laboratories) that were activated by washing three times with cold bead activation buffer (20âmM HEPES pHâ7.9, 10âmM KCl, 1âmM CaCl2, 1âmM MnCl2). ConA bead/cell mixtures were resuspended in cold antibody buffer (20âmM HEPES pHâ7.5, 150âmM NaCl, 0.5âmM spermidine, 1à Roche cOmplete, Mini, EDTA-free protease inhibitor, 0.01% digitonin, 2âmM EDTA), then incubated with 0.5âμg primary antibodies: H3K27me3 (CST, 9733) and H3K4me3 (CST, 9733) or IgG (Epicypher) overnight in a 4â°C cold room. Unbound antibodies were washed three times each with cold digitonin buffer (20âmM HEPES pHâ7.5, 150âmM NaCl, 0.5âmM spermidine, 1à Roche cOmplete, Mini, EDTA-free protease inhibitor, 0.01% digitonin). ConA bead/cell mixtures were then resuspended in 50âμl cold digitonin buffer and incubated with our homemade pAG-MNase in a 4â°C cold room for an hour on a nutator. Unbound pAG-MNase was washed three times with cold digitonin Buffer. MNase was activated by addition of CaCl2 and incubated in a 4â°C cold room for 30âmin on a nutator to cleave and release antibody-bound chromatin. The reaction was stopped by adding cold stop buffer (340âmM NaCl, 20âmM EDTA, 4âmM EGTA, 50âμgâmlâ1 RNase A, 50âμgâmlâ1 glycogen, 1âpgâμlâ1 E. coli spike-in DNA). Cleaved chromatin was then released by incubating at 37â°C for 10âmin. CUT&RUN enriched DNA in the supernatant can be collected using magnetic beads and purified using the Monarch PCR and DNA cleanup kit (NEB T1030L). CUT&RUN libraries were prepared with 10âng CUT&RUN DNA using NEBNext Ultra II DNA library prep kit (NEB), according to manufacturerâs protocol. Libraries were sequenced on the Illumina NextSeq 2000 system, 2âÃâ50âbp paired-end reads.
Paired-end fastq files were aligned to hg38 reference genome using Bowtie2 with the settings â–very-sensitive –no-mixed –no-discordant –phred33 -I 10 -X 700â72. Sequencing reads were also aligned to the E. coli genome to map spike-in reads. For spike-in normalization, the total number of mapped reads to E. coli genome was used to calculate normalization factor for CUT&RUN samples. SAM files were converted to BAM files using samtools73. Bigwig files were generated from BAM files using Deeptools74. Genome browser tracks of big files were generated using the Integrative Genomics Viewer (IGV)75. Peaks were called using MACS2 using callpeak function with â-f BAME -keep-dup 1 -q 0.05â and IgG was used as a control76. DeSeq2 analysis from DiffBind R package with option âspikein=Trueâ was used to compare differential binding between conditions (FDRâ<â0.1)77.
RTâqPCR
RNA was isolated using the RNeasy Plus kit (74134, Qiagen). RNA was reverse transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (4368814, Thermo Fisher Scientific). qPCR was completed using PerfeCTa SYBR Green SuperMix Reaction Mixes (95054-500, QuantaBio) and the primers listed below. Cq values were normalized to STAU1 as a reference gene.
CyCIF analysis
Human breast cancer tissue array BRC482 was purchased from Pantomics and consists of 16 cases, with one normal breast tissue core paired with two tumour tissue cores from each patient. FFPE sections of PDX samples and a tissue microarray were prepared and stained with a 23-plex and 33-plex antibody panel, respectively, using tissue-based cyclic immunofluorescence (CyCIF) as previously outlined. Baking and dewaxing: to prepare the samples for antibody staining, tissue sections on glass slides underwent an automated process facilitated by the Leica Bond RX machine. The protocol started with a 30âmin baking step at 60â°C. Subsequently, the slides were subjected to dewaxing at 72â°C in BOND Dewax Solution, followed by an antigen-retrieval step conducted at 100â°C for 20âmin using BOND Epitope Retrieval Solution 1 (ER1). Photo-bleaching and autofluorescence reduction: after the baking and dewaxing steps, the slides were immersed in a bleaching solution (4.5% H2O2, 20âmM NaOH in 1à PBS) and exposed to LED light for 1âh to mitigate autofluorescence. Excess bleaching solution was removed by washing the slides six times with 1à PBS (10â15âs each). Subsequently, the slides were incubated overnight at 4â°C in darkness with secondary antibodies diluted in SuperBlock Blocking Buffer (1:1,000; Thermo Fisher Scientific, 37515). After this incubation, the slides were washed six times with 1à PBS and were photobleached again for 1âh. Antibody staining, coverslip mounting and imaging: for each CyCIF cycle, the samples were incubated overnight at 4â°C in the absence of light with Hoechst 33342 (1:10,000; Thermo Fisher Scientific, 62249) for nuclear staining. Simultaneously, primary antibodies were introduced, which were either conjugated or unconjugated, diluted in SuperBlock Blocking Buffer (Thermo Fisher Scientific, 37515). In cases in which primary antibodies were unconjugated, subsequent incubation with secondary antibodies was performed at room temperature for 1âh in the dark. After staining, the slides underwent a series of six 10âs washes with 1à PBS, and then they were mounted with 24âÃâ60âmm coverslips, using 80â100âµl of 50% glycerol, and left to dry at room temperature for 30âmin. Once coverslipped, automatic imaging was conducted using the RareCyte Cytefinder II HT system with the following channels: UV, cy3, cy5 and cy7. Imaging parameters remained consistent as follows: binning: 1âÃâ1 for PDX whole-slide images; binning 2âÃâ2 for the TMA; objective: Ã20; numerical aperture: 0.75; resolution: 0.325âmmâpxâ1. Image exposures were fine-tuned for each channel to prevent signal saturation but were maintained uniformly across samples. Between CyCIF cycles, the coverslips were demounted by immersing slides in containers of 1à PBS (5 slides per container) for 10âmin at room temperature. Before initiating the subsequent antibody staining cycle, slides were photobleached for 1âh to deactivate the fluorophores and washed again in PBS six times to remove residual bleaching solution. The acquired images were processed using the Docker-based NextFlow pipeline MCMICRO. Initially, raw images from the Cytefinder II HT underwent illumination correction via the BaSic module. Subsequently, they were stitched and registered using the ASHLAR module, resulting in the generation of assembled OME.TIFF files for each slide. Full code is available at GitHub (https://github.com/labsyspharm/mcmicro).
Antibodies were as follows: CK14 (Abcam, ab77684, LL002, conjugated to FITC, 1:500), CK8 (eBioscience, 11-9938-80, LP3K, conjugated to FITC, 1:400), pan-CK (eBioscience, 53-9003-82, AE1/AE3, conjugated to AF488, 1:1,000), EZH2 (CST, 45638, D2C9, conjugated to AF647, 1:100), cPARP (CST, 6987S, D64E10, conjugated to AF647, 1:100), E-cadherin (CST, 9835S, 24E10, conjugated to AF647, 1:600), anti-rabbit IgG (Invitrogen A-31572, conjugated to AF555, 1:1,000) and anti-mouse IgG (Invitrogen, A-31571, conjugated to AF647, 1:1,000).
ERV expression
To count reads for ERVs, ERV locus information was collected from the ERVmap database78. Salmon (v.0.14.1)79 was used to create a customized reference transcriptome set that includes human genome transcriptome (hg38) and ERVmap database. Using the database, RNA-seq data of MDA-MB-468 cells treated with DMSO or EZH2i tazemetostat were quantified by Salmon. DESeq2 was used for counts normalization and differential expression analysis.
Oligonucleotides
Primers for RTâqPCR were as follows in 5â²â3â² orientation: STAU1 (fwd: GGATGAGTTCAGGATGCCTTAT, rev: GGTGTGATGTCCTTGACTAACT), BMF (fwd: ACTTCAGCTCTTCCCTCTCA, rev: GAGTCTGGGTAGCTTTGTCTTC), GATA3 (fwd: CTCATTAAGCCCAAGCGAAG, rev: GTCTGACAGTTCGCACAGGA), FOS (fwd: GTCTTCCTTCGTCTTCACCTAC, rev: GAGTCAGAGGAAGGCTCATTG), CYBRD1 (fwd: AGATCCTGCATACAGTACATTCC, rev: CATTGCGGTCTGGTGACTAT), PDK4 (fwd: GCCTTCCCTTACACCAATAGAG, rev: GTTGGTGCAGTGGAGTATGT), SCNN1A (fwd: GGCTGTGCCTACATCTTCTATC, rev: GAGAAGTCAACCTGGAGCTTATAG), STAT5A (fwd: GCCACCATCACGGACATTAT, rev: CAAACTTGGTCTGGGTCTTCA), IGFBP5 (fwd: GAAGAAGGACCGCAGAAAGAA, rev: CTCAGACTCCTGTCTCATCTCA), TNFRSF21 (fwd: CCAACTCTTCTGCCTCTGTTAG, rev: GAGGGTCTTGTTCACGTCTTC), GDA (fwd: CGCACACTGTCCCAATTCTA, rev: TCTGTACCCAGCCCTATCTT). Primers for ChIPâqPCR were as follows in 5â²â3â² orientation: GATA3 promoter (fwd: TTGGTGCTCGCGATTGAA, rev: AAATGCTGACTTCTGAGGCTAA), GATA3 enhancer (fwd: ATCCATCAGCCCTTCTTTCTG, rev: GCGCCATCTACTGGGTTATT).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.