Fly stocks and husbandry
Flies were maintained at 25â°C and 50â65% relative humidity under a 12âh light/12âh dark cycle. D. melanogaster stocks Canton-S (catalogue no. 64349), UAS-GCaMP6s (42746 and 42749), UAS-mCD8::GFP (5137), 71G01-Gal4 (39599), fruLexA (66698) and UAS-CsChrimson-mVenus (55134 and 55136) were obtained from the Bloomington Stock Center. LexAop-SPA-T2A-SPA was generated in a previous study19. D. yakuba Ivory Coast (14021-0261.00), D. erecta (14021-0224.01) and D. ananassae (14024-0371.34) were obtained from the Cornell (formerly UCSD) Stock Center. D. eugracilis (SHL12) was obtained from the Kyoto Stock Center. Drosophila suzukii (WT3) (L. Zhao, Rockefeller); D. melanogaster SplitP1-Gal4 (D. Anderson, Caltech); D. melanogaster fruGal4 and fruAD/fruDBD (B. Dickson, Janelia Research Campus); D. melanogaster ppk23-Gal4 (K. Scott, UC Berkeley); D. yakuba fruGal4, fruDBD and fruAD inserts62, 71G01-Gal4 (ref.â63), UAS-GCaMP6s, UAS-CsChrimson, UAS-Kir2.1 and attp1730 (D. Stern, Janelia Research Campus); D. erecta 71G01-Gal4 (insertion no. 5), D. erecta UAS-GCaMP6s (insertion no. 5), D. erecta UAS-CD8::GFP (insertion no. 2), D. erecta UAS-CsChrimson::tdTomato (insertion no. 1), D. yakuba dsxGal4 and dsxAD inserts64 (Y. Ding, University of Pennsylvania); D. yakuba UAS-mCD8-GFP (insertion nos. 2 & 3), UAS-GFP (insertion no. 8), UAS-SPA-GFP (insertion no. 2), ppk23-Gal4 (insertion no. 2), ppk23 mutant, ppk25 mutant and Or67d mutant were generated in this study. See Supplementary Table 1 for detailed genotypes by figure.
Construct design and generation
The ie1.mCherry and ie1.eGFP minigene reporters used as transformation markers were constructed from the following fragments: the ie1 promoter was PCR amplified from pBac{orco.QF, QUAS.GCaMP, ie1. DsRed} (gift from D. Kronauer), Drosophila codon-optimized mCherry and eGFP gene blocks were synthesized (IDT), and the p10-3â² untranslated region (UTR) was PCR amplified from pJFRC81 (Addgene, 36432). These were then cloned by Gibson assembly (NEB) into pBac{3XP3-EGFP, Pactin-Ptrsps} (Addgene, 86861) after digest with BstZ17I and Bsu36I to generate pRC7a (ie1.mCherry) and pRC10a (ie.eGFP).
To create a more flexible multicloning site in pRC10a, a gBlock (IDT) containing the following restriction enzyme sites, HpaI, HincII, HindIII, KpnI, NotI, XhoI, XbaI and FseI, was synthesized and cloned by Gibson assembly into pRC10a. The resulting vector was pIM145. mCherry was then cloned with NheI and HpaI from pRC7a into the pIM145 backbone in place of GFP to generate pIM148.
To generate a P1 driver line, the 3,844âbp fragment from Vsx2 gene that is present in pGMR71G01 was amplified from D. melanogaster genomic DNA. This was then cloned by Gibson assembly into the backbone of pIM145 in front of a Gal4 gene block (IDT), generating pRC12b.
To generate a BAC vector containing UAS-GFP, the following cassette, 10âÃâUAS-IVS-Syn21-GFP p10 UTR, was excised from pJFRC81 (Addgene, 36432) with HindIII and FseI and ligated into pIM145. The resulting vector was pIM153.
To generate a BAC vector containing UAS-SPA-GFP, a Drosophila codon-optimized SPA-GFP was synthesized as a gBlock (IDT) and cloned by Gibson assembly (NEB) into pIM147 in place of GCaMP6s to generated pIM141.
To generate ppk23-Gal4, a DNeasy Blood & Tissue kit (Qiagen) was used to purify wild-type D. yakuba genomic DNA. First, 50âmg of flies were collected and snap-frozen in liquid nitrogen. Flies were ground to a fine powder using a mortar and pestle. Powder was transferred to a microcentrifuge tube and processed according to the DNeasy Blood & Tissue kit instructions. The resulting genomic DNA was used as a template for PCR amplification of the ppk23 promoter. The 2.7âkb promoter was cloned into pRC12B to generate pIM169.
To generate a BAC vector containing a blue fluorescent marker for visualization, a Drosophila codon-optimized mTagBFP2 was synthesized. Owing to the sequence complexity of mTagBFP2, it was designed as two overlapping gBlocks (IDT) and included a portion of the ie1 promoter for seamless assembly into the destination vector. mTagBFP2 was cloned by Gibson assembly (NEB) into pIM148 in place of mCherry to generate pIM149.
CRISPRâCas9-mediated deletion of ppk23 in D. yakuba
Two single guide RNAs (sgRNAs) were designed to direct Cas9-mediated cleavage to the first exon and 3â² UTR of the ppk23 gene locus in D. yakuba. gRNA off-target potential was determined using CRISPR optimal target finder (http://tools.flycrispr.molbio.wisc.edu/targetFinder/index.php). sgRNA sequences, sgRNA1 CATCGGTGCGGTCACCGCAC and sgRNA2 GTGTTGCATACTTAGCGGCG, were PCR amplified with Q5 High-Fidelity master mix (NEB) and cloned into pCFD4 (Addgene, 49411) by Gibson assembly (NEB). The resulting vector was pIM179. pIM148 was digested with HpaI and SmaI to liberate ie1p mCherry p10 UTR. The mCherry cassette was then ligated to pDsRed-attP (Addgene, 51019) which was digested with AgeI and BsiWI to remove the 3âÃâP3-DsRed cassette then Klenow end-filled. This generated pIM174. The 1âkb homology arms beginning at the predicted Cas9 cut sites in ppk23 were PCR amplified with Q5 High-Fidelity master mix (NEB) and cloned into pIM174. The resulting vector was pIM175. A cocktail of pIM179, pIM175 and Cas9 protein was injected into wild-type D. yakuba embryos by Rainbow Transgenic Flies using standard injection procedures. Viable G0 flies were mated to wild-type male or virgin female flies. F1 progeny were screened visually by mCherry expression. mCherry-positive F1s were individually crossed to wild-type male or virgin female flies then killed for genomic DNA. Deletion of ppk23 was confirmed in mCherry-positive F1s by genotyping using primers internal to and flanking the targeted genomic region. PCR products from genotyping were sequenced to verify the exact genome modification. mCherry-positive F2s from sequence-verified F1s were self-mated and mCherry-positive F3 virgin females were genotyped by non-lethal methods to identify females homozygous for ppk23 deletion. As the ppk23 locus is on the X chromosome, virgin females homozygous for the ppk23 null mutation were then mated to males hemizygous for the mutation to produce a stable line.
CRISPRâCas9-mediated deletion of ppk25 in D. yakuba
Two sgRNAs were designed to direct Cas9-mediated cleavage to the first exon and 3â² UTR of the ppk25 gene locus in D. yakuba. gRNA off-target potential was determined using CRISPR optimal target finder. sgRNA sequences, sgRNA1 GUCGGUCGAUGCAACCGGAC and sgRNA2 UAAACUUAACAACAUCGGAG, were synthesized from Synthego. The 1âkb homology arms beginning at the predicted Cas9 cut sites in ppk25 were ordered as gBlocks from IDT. The ppk25 start code in the 5â² homology arm was mutated from ATG to TTG. The homology arms were cloned sequentially into pIM174 by Gibson assembly. The resulting vector was pIM188. A cocktail of pIM188, sgRNA1, sgRNA2 and Cas9 protein was injected into wild-type D. yakuba embryos by Rainbow Transgenic Flies using standard injection procedures. Viable G0 flies were mated to wild-type male or virgin female flies. F1 progeny were screened visually by mCherry expression. mCherry-positive F1s were individually crossed to wild-type male or virgin female flies then killed for genomic DNA. Deletion of ppk25 was confirmed in mCherry-positive F1s by genotyping using primers internal to and flanking the targeted genomic region. PCR products from genotyping were sequenced to verify exact genome modification. mCherry-positive F2s from sequence-verified F1s were self-mated and mCherry-positive F3 virgin females and males were genotyped by non-lethal methods to identify flies homozygous for ppk25 deletion.
CRISPRâCas9-mediated deletion of Or67d in D. yakuba
Two sgRNAs were designed to target 22âbp downstream of the start codon (sgRNA1) and 392âbp (sgRNA2) downstream of the stop codon of D. yakuba Or67d, removing a total of 1,783âbp of endogenous DNA. Off-targets were determined using CRISPR optimal target finder. sgRNA1 (GACUUUACGAAAGCGCUCCA) and sgRNA2 (ACUGCUGCUGUCCAAAGGAG) were synthesized by Synthego. A 1,035âbp 5â² homology arm was amplified from D. yakuba genomic DNA using Q5 High-Fidelity master mix (NEB) and cloned into pIM174 using XmaI and NdeI restriction enzymes. A 1,174âbp 3â² homology arm of was amplified from D. yakuba genomic DNA using Q5 High-Fidelity master mix (NEB) and cloned into pIM174 using AvrII and XhoI restriction enzymes. The resulting vector was pGK1. A cocktail of pGK1, sgRNA1/2 and Cas9 protein was injected into wild-type D. yakuba embryos by Rainbow Transgenic Flies using standard injection procedures. Viable G0 flies were mated to wild-type male or virgin female flies. Progeny were screened, mated and genotyped as described for other mutants to produce a stable line.
Immunohistochemistry
Adult brains were dissected in Schneiderâs media (Sigma) then immediately transferred to cold 1% PFA (Electron Microscopy Sciences) and fixed overnight at 4â°C. Following overnight incubation samples were washed in PAT3 buffer (0.5% BSA/0.5% Triton/PBS pH 7.4) three times. Brains were blocked in 3% Normal Goat Serum for 90âmin at room temperature. Primary antibodies 1:1,000 chicken anti-GFP (Abcam, ab13970), 1:50 mouse anti-brp (Developmental Studies Hybridoma Bank nc82), 1:2,000 rabbit anti-FruM (generated for this study by YenZyme against a synthesized peptide: HYAALDLQTPHKRNIETDV70) and 1:500 guinea pig anti-FruM (gift from D. Yamamoto, Tohoku University) were incubated for 3âh at room temperature then for 2â3âd at 4â°C. Brains were washed extensively in PAT3 buffer. Secondary Alexa Fluor antibodies (Life Technologies) were incubated for 3âh at room temperature then for 2â3âd at 4â°C. Brains were washed three times in PAT3 buffer then once in PBS. Samples were mounted in Vectashield (Vector Laboratories). Images were captured on a Zeiss LSM 880 using a Plan-Apochromat Ã20 (0.8 numerical aperture) objective.
Leg images were taken using the native fluorescence of animals expressing UAS-CD8::GFP (insertion no. 2). Animals were aged approximately 3â5âdays and legs were mounted in Vectashield and femurs stabilized using ultraviolet glue. Images were taken at Ã25 with Ã1.6 digital zoom.
For analysis of P1 projections, images were registered to the JRC2018M template brain using the Computational Morphometry Toolkit (https://www.nitrc.org/projects/cmtk/) and P1 neurons were segmented in VVD Viewer (https://github.com/JaneliaSciComp/VVDViewer).
Courtship assays in the light
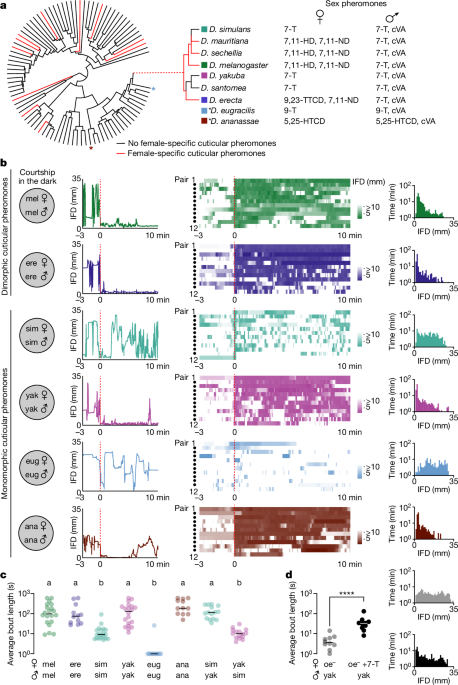
All single choice assays were conducted at 25â°C, 50â65% relative humidity between 0 and 3âh after lights on. Male flies were collected shortly after eclosion and group housed for 4â7âdays before assay. Target virgin females were 4â7âdays post-eclosion. Assays were performed in 38-mm-diameter, 3-mm-height circular, slope-walled chambers in a 4âÃâ4 array back-lit using a white light pad (Logan Electric). Fly behaviour was recorded from above the chambers using a PowerShot SX620 camera (Canon) or Point Grey FLIR Grasshopper USB3 camera (GS3-U3-23S6M-C: 2.3 MP, 162 FPS, Sony IMX174, Monochrome). A virgin female was transferred to the chamber by mouth aspiration followed by a test male. Once the male was loaded into the chamber the assay commenced and the activity of the flies was recorded for 10âmin. Note that courtship assays in the light were not conducted on food to avoid flies copulating too quickly under these conditions. In the absence of food, females generally move much quicker. This results in shorter male courtship bouts, probably explaining the difference in bout lengths observed between males in the dark on food compared with males in the light off food (that is, bouts were generally longer in the dark when on food).
Courtship assays in the dark
Courtship assays performed in the dark were conducted at 25â°C, 50â65% relative humidity between 0 and 3âh after lights on. Male flies were collected shortly after eclosion and group housed for 4â7âdays before assay. Target virgin females were 4â7âdays old. Assays were performed on food in 35âÃâ10âmm2 Petri Dishes (Falcon). Females were transferred to the chamber by mouth aspiration followed by a test male. Once the male was loaded into the chamber the assay commenced and the activity of the flies was recorded for 3âh. The extended recording period was necessary due to the lengthy latency to courtship initiation in the dark, where males must discover females by chance. A manual observer recorded the time of courtship initiation for all videos, and the subsequent 10âmin were used for analysis. Assays were back-lit by infrared light-emitting diode (LED) strips (940ânm, LED Lights World). Fly behaviour was recorded from above the chambers using a Point Grey FLIR Grasshopper USB3 camera (GS3-U3-23S6M-C: 2.3 MP, 162 FPS, Sony IMX174, Monochrome) using the Flycapture2 Software Development Kit (v.2.13.3.61) (FLIR).
Courtship quantification
For all behavioural experiments, variance was assessed by preliminary study and predetermined sample size were chosen. Collection of experimental and control animals was randomized with roughly equal numbers collected on the same days. Courtship was scored either by automated tracking using machine vision (Matlab, FlyTracker, Caltech) or manually by an observer blind to the experimental set-up (for example, species, genotype, perfume-treatment). For manual analysis orienting, wing extension, chasing, mounting and copulation were used to score courtship behaviour. To facilitate plotting on a log scale all 0 values were changed to 0.1. For automated courtship scoring, an 8âmm IFD threshold was found to most accurately capture the courtship bouts scored by the blind observer, producing the lowest number of false positives and negatives. Unfortunately, variations in the food and/or infrared lighting meant that for approximately half of the videos collected we could not obtain high-quality tracking data. Thus, we opted to track a random subset, that is, the first 12 consecutive courting pairs from which quality tracking was possible. This provides a representative visual assessment of courtship dynamics, whereas the complete dataset of all courting pairs was scored manually by blinded observer. Extended Data Fig. 1 illustrates that although IFD tracking and manually scored datasets differed in the absolute quantity of courtship scored, the overall trends in the data were consistent. However, because false detections in the automatically scored data compress the dynamic range of the data, we believe that bout length is the most sensitive indicator of pheromone-dependent courtship in assays conducted in the dark, effectively capturing a maleâs ability to persistently pursue a female, guided by ongoing pheromonal feedback. Consequently, bout length scored by a blinded observer is used in all figures. The only exceptions to using this metric throughout our study are: (1) in the initial analysis of courtship in the dark (Fig. 1), where automatically quantified IFD is compared with these manually scored courtship metrics (Extended Data Fig. 1) before selecting blinded, manually scored bout length as the most accurate; (2) in the optogenetic analysis of P1 subtypes in D. melanogaster and D. yakuba, for which it was important to provide a more granular description of courtship dynamics (Fig. 4a,b and Extended Data Figs. 6a,b and 7), and thus JAABA (Janelia) behavioural classifiers were employed to quantify courtship pursuit and song; and (3) in the extended data, where in addition to bout length, more common measurements of courtship (for example, latency to court, total percentage time courting) were plotted to provide a comprehensive view (Extended Data Figs. 3bâd, 4b and 8a,c,d,f,g).
Generation of oeâ animals
Oenocyte ablation was performed genetically by crossing male +;PromE(800)-Gal4, tub-Gal80TS;+ flies to female +;UAS-StingerII, UAS-hid/CyO;+ at 18â°C. Newly eclosed virgin females were collected and kept at 25â°C for 1âd. Females were then shifted to 30â°C for 2âd and then allowed to recover at 25â°C for 2âd before use in experiments. Females were screened for GFP expression to confirm oenocyte ablation12.
Perfuming
Mock, 7-T and 7,11-HD perfuming of oeâ or D. yakuba females was performed by adding heptane, 2âµg of 7-T or 2âµg of 7,11-HD (Cayman Chemicals), respectively, to 1âml of heptane in a 32âmm scintillation vial (ThermoScientific). A vacuum was then applied to evaporate the heptane from the vial. We added 5â8 flies to each vial and vortexed at low speed for 30âs, three times. cVA perfuming was perfumed by dissolving cVA to a concentration of 5âmgâmlâ1 in ethanol. Then, 0.5âµl was then applied directly to each flyâs abdomen by pipette. All flies were returned to food to recover for at least 1âh before experimentation.
Photoactivation
For photoactivation experiments fruGal4 was used to express SPA-GFP. Photoactivation was performed on adult flies aged 24â48âh after eclosion. Brains were imaged at 925ânm to identify suitable sites for photolabeling while not stimulating photoconversion. Using PrairieView, a region of interest (ROI) was then drawn around projections unique to the cell type of interest and photoconversion was stimulated in a single z-plane by brief exposure of the ROI to 710ânm laser light. Power was 5â35âmW at the back aperture of the objective, depending on the depth of the neurite being converted. This process was repeated 50â100 times, interposed with rests to allow for diffusion of the photoconverted molecules, until the cell type of interest was uniformly above background levels of fluorescence.
Functional imaging
All imaging experiments were performed on an Ultima two-photon laser scanning microscope (Bruker) equipped with galvanometers driving a Chameleon Ultra II Ti:Sapphire laser. Emitted fluorescence was detected with GaAsP photodiode (Hamamatsu) detectors. All images were collected using PrairieView Software (v.5.5) at 512âÃâ512 pixel resolution. Fluorescence time-series were extracted using FIJI (v.2.14.0/1.54f). Ventral nerve cord and LPC preparations were performed as previously reported13,19. For all imaging experiments, the presentation order of female or male stimuli was randomized and the strongest responding stimulus was presented again the end of an experiment to confirm the continued health of experimental male. Sample sizes were not predetermined.
For the D. yakuba P1 imaging, âsplitP1â (71G01-AD 15A01-DBD intersection) had to be used rather than 71G01-Gal4 because this line was weak and bleached before an experiment could be completed. These splitP1 animals showed inter-animal variability in responses. Only about 1 in 5 (5 of 27) animals exhibited P1 responses to conspecifics, although in responding animals the responses were uniform and predictably evoked each time a male tapped a conspecific female (as shown in Fig. 2). We discovered this probably resulted from stochastic labelling of the FruâDsx+ P1 subset. Specifically, splitP1 predominantly labels Fru+ P1 neurons, and labels 2â3 FruâDsx+ neurons in only some animals, as previously found in D. melanogaster65. Indeed, when we subsequently imaged Dsxâ©P1 (71G01-DBD dsx-AD intersection), no inter-animal variability was observed and all animals responded to conspecifics with each tap. The same was found to be true when imaging for all Dsx+ projections in the LPC. Therefore, for simplicity and understanding we have presented just responding D. yakuba splitP1 males in Fig. 2b.
Imaging analysis
For each GCaMP recording, an ROI was drawn in the LPC neuropil where axonal projections are densest for P1 (indicated in Fig. 2a) or in the ventral nerve cord neuropil where Ppk23+ neurons have their sensory afferents (indicated in Fig. 3a). For all experiments, 3â5âs were recorded before the stimulus presentation to create a baseline. Twenty frames (approximately 2âs) of this pre-stimulus period were then averaged to determine baseline fluorescence (F0), and âF/F0 was calculated as ÎFt/F0â=â(FtâââF0)/F0, where t denotes the current frame.
Optogenetic set-up
Optogenetic assays were performed in a 38âmm diameter, 3âmm height circular chamber with sloping walls. The chamber was placed in the middle of a 3-mm-thick acrylic sheet suspended on aluminium posts above a 3âÃâ4 array of 627ânm LEDs (Luxeon Star LEDs). LEDs were attached to metal heat sinks (Mouser Electronics) which were secured at 5âcm intervals to a 30âÃâ30âcm2 aluminium wire cloth sheet (McMaster-Carr). LEDs were driven by Recom Power RCD-24-0.70/W/X2 drivers, which were powered by a variable DC power supply. Infrared LED strips (940ânm, LED Lights World) attached to the wire cloth between the heat sinks provided back-illumination of the platform. LED strips were covered with 071 Tokyo blue filter (Lee Filters) to remove potential activating light emitted from the illumination source. LED drivers were controlled by the output pins of an Arduino running custom software. Fly behaviour was monitored from above the chamber using a Point Grey FLIR Grasshopper USB3 camera (GS3-U3-23S6M-C: 2.3 MP, 162 FPS, Sony IMX174, Monochrome) outfitted with 071 Tokyo blue filter (Lee Filters) to avoid detection of light from the high-power LEDs. Flies were recorded at 30 frames per second. Custom software was used for data acquisition and instrument control during assays. Light intensity was measured with a photodiode power sensor (Coherent, 1212310) placed at the location of the behavioural chamber. The peak wavelength of the LED (627ânm) was measured across a range of voltage inputs. Measurements were repeated three times and averaged. The baseline intensity before LED illumination was subtracted.
Optogenetic assays
Flies were reared on standard SY food in the dark at 25â°C and 50â65% relative humidity. P1>UAS-CsChrimson and UAS-CsChrimson control male flies were collected shortly after eclosion, group housed for 3âd, then shifted to food containing 0.4âmM all trans-retinal (Sigma) for 48âh before being assayed. ppk23-Gal4>UAS-CsChrimson and UAS-CsChrimson control male flies were collected shortly after eclosion, group housed for 3âd, then shifted to food containing 0.4âmM all trans-retinal for 24âh before being assayed. Fruâ©P1>UAS-CsChrimson, Dsxâ©P1>UAS-CsChrimson and UAS-CsChrimson control males were collected shortly after eclosion, grouped housed for 2âd and then shifted to food containing 0.4âmM all trans-retinal for 24âh (Fruâ©P1) or 48âh (Dsxâ©P1) before being assayed. Target virgin females were 4â6âd old. Flies were added to a 38âmm diameter, 3âmm height circular courtship chamber by mouth aspiration. Once a male was transferred to a courtship chamber containing a virgin female, the assay commenced and the activity of the flies was recorded for 10âmin. Neurons expressing CsChrimson were activated by 627ânm wavelength LED stimulation. To activate splitP1 (w; 71G01-AD/+; 15A01-DBD/UAS-CsChrimson) neurons in D. yakuba the following stimulation protocol was used: 2âmin dim white light followed by 2âmin 627ânm LED (5âHz, 100âms pulse-width, 10âµWâmmâ2) alternating for 10âmin total. For activation of P1 (UAS-CsChrimson::tdTomato/+; 71G01-Gal4/+) neurons in D. erecta the stimulation protocol was: 2âmin dim white light followed by 2âmin 627ânm LED (5âHz, 100âms pulse-width, 3.4âµWâmmâ2) alternating for 10âmin total. To activate Ppk23+ neurons in D. yakuba the following stimulation protocol was used: 2âmin dim white light followed by 2âmin 627ânm LED (5âHz, 100âms pulse-width, 8âµWâmmâ2) alternating for 10âmin total. All assays were conducted at 25â°C, 50â65% relative humidity between 0 and 3âh after lights on. To activate Fruâ©P1 and Dsxâ©P1 neurons in D. yakuba the following stimulation protocol was used: 2âmin dim white light followed by 2âmin 627ânm LED (5âHz, 100âms pulse-width, 14âµWâmmâ2 for Fruâ©P1 and 37âµWâmmâ2 for Dsxâ©P1) alternating for 10âmin total. The 48âh retinol stimulation protocol was used in D. melanogaster males for each P1 population, and the light intensity used was 3.4âµWâmmâ2. A blinded observer scored orienting, wing extension, chasing, mounting and copulation to quantify courtship behaviour for the purposes of calculating overall courtship indices. To quantify dynamic behaviours, videos were tracked using FlyTracker (Caltech). Behavioural classifiers for courtship pursuit behaviour (consisting or orienting towards the female and moving towards her) and unilateral wing extensions were then trained using JAABA (Janelia) in each species.
Statistical analysis
Statistical analyses were performed in GraphPad Prism 9. Before analysis, normality was tested for using the ShapiroâWilk method for determining whether parametric or non-parametric statistical tests would be used. In cases for which multiple comparisons were made, appropriate post hoc tests were conducted as indicated in the figure legends. All statistical tests used were two-tailed. Experimenters were blind to experimental conditions during analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.