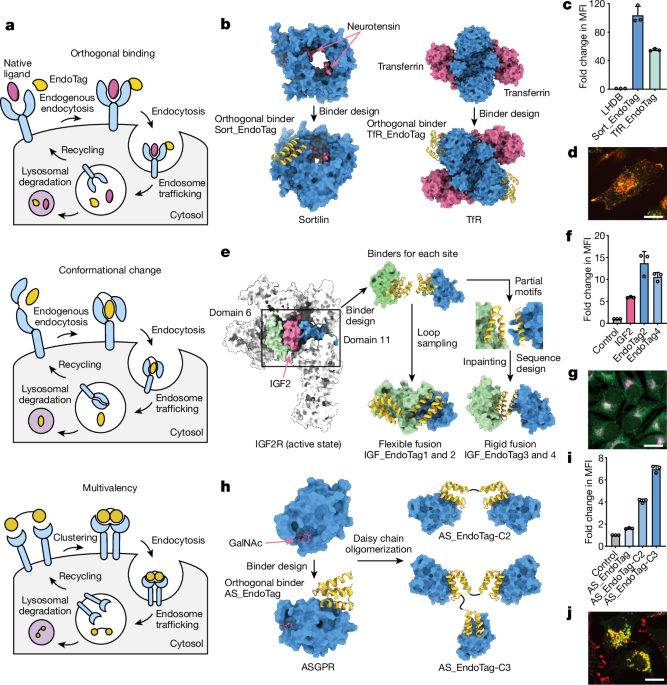
Computational design of sortilin minibinders as Sort_EndoTags
Using a Rosetta-based binder design protocol15, 21,000 binders were generated to each of 2 sites on the sortilin. The epitope of site1 comprises five amino acids (UniProt numbering: F92, V93, T546, T559 and T561), chosen since it provided a modest patch of exposed hydrophobicity while avoiding any sites of known interactions. The selected epitope has the added feature that a binder to this location would be pH-dependent, since this region undergoes considerable structural change at low pH18. As previously described15, we used a set of scaffold libraries to generate several million docks to each of the sites. As in the protocol, 100,000 docks were sub-selected and sequence was designed. Helical motifs were extracted, and 3,000 designs were selected, grafted and subjected to further design. Designs were filtered based on their Rosetta ddG and ContactMolecularSurface to the hydrophobic residues listed above. This resulted in 42,000 designs that were tested experimentally. The final designed sequences for Sort_EndoTag are provided in Supplementary Table 2.
Computational design of IGF2R and ASGPR minibinders
The minibinders against IGF2R domain 6 and domain 11 were computationally designed via a Rosetta-based approach as described15. In brief, the structures of IGF2R domain 6 (Preotein Data Bank (PDB) 6UM2) and IGF2R domain 11 (PDB 1GP0) were refined using Rosetta Fastrelax with coordinate constraints. The residues at the IGF2-binding site for each domain were selected as âhotspotâ residues. Helical protein scaffolds were docked against the hotspot residues via the Patchdock followed by the Rifdock protocol. After sequence optimization with Rosetta FastDesign and filtering with Rosetta interface metrics including ddg and contact_molecular_surface, the top candidates were then resamplered with Rosetta Motifgraft44 and FastDesign. Candidates passing previous filters were then filtered again with exposed hydrophobicity (sap_score) and optimized with a net-charge of â7. The final designed sequences for IGF_EndoTag are provided in Supplementary Table 1.
The minibinders against ASGPR were designed with a Rosetta-based approach integrated with ProteinMPNN and AlphaFold2. The crystal structure of ASGPR (PDB 5JQ1) was refined and helical protein scaffolds were docked against the exposed hydrophobic residues via Patchdock followed by Rifdock. The sequences were optimized with protein-MPNN and interface scores were calculated with Rosetta Fastrelax. The models were then predicted by AlphaFold2 and scored after Fastrelax. Designs with pae_interaction<10 and relaxed_ddgâ<ââ40 were selected for resampling with another round of protein-MPNN prediction followed by Rosetta Fastrelax. After final round filtering with pae_interaction, relaxed_ddg and sap_score, the sequences were further optimized to have a net-charge of â7. The final designed sequences for AS_EndoTag are provided in Supplementary Table 4.
Computational design of IGF_EndoTags
To generate flexible IGF2R agonists, all combinations of GS linkers with various lengths linking D6mb and D11mb were modelled with AlphaFold225. The designs with poor monomer plddt (plddtâ<â85) were dropped.
To generate rigid IGF_EndoTags, the major binding helix from D11mb or the native IGF2-binding helix, and two interface helices from D6mb were extracted. Crystal structures obtained for D6mb and D11mb in complex with IGF2 and IGF2R were used as starting points for design. Domains 6 and 11 of the complex structures were aligned with the respective domains of IGF2R in the putative receptor internalizing conformation available in the Protein Data Bank (PDB: 6UM2). In this orientation, the two interface helices from D6mb and the single interface helix from D11mb were extracted and used as motifs to scaffold by protein inpainting24. To increase the likelihood of design success, the D11mb structure was adjusted to form an ideal three helical bundle with the two domain 6 helices. Protein inpainting was implemented such that the interacting residues within 3âà of the receptor maintained the same identity as in the original minibinders. To increase design diversity, the domain 11 helix motif was randomly perturbed by rigid-body translations (up to 5âà ) and rotations (up to 10 radians) for each design prior to inpainting a scaffold between the motifs. The best inpainting outputs were selected by RosettaFold LDDT metrics (>0.5) for the inpainted region and used for sequence design with ProteinMPNN. ProteinMPNN sequence design was performed on the inpainted outputs in their desired complex orientation (with both domains 6 and 11 present) while fixing the original minibinder identities of interface residues (D6mb: R4, V8, Q11, D15, V20, K24, M25, I27, I31 and E34; D11mb: M1, A4, L7, L8 and W11). After 2,000 sequences were generated for each ProteinMPNN input, designs were filtered by predicted Rosetta ddG. AlphaFold2 structure predictions of the designed sequences were filtered by the pLDDT metric (keeping those with pLDDTâ>â90), and designs with a sub-angstrom backbone atom root mean squared deviation to the original design models realigned to D6mb and D11mb crystal structures (in complex with the IGF2âM6PR target domains). Finally, the complexes were assessed by Rosetta FastRelax. Designs with ddG metrics less than â40 and spatial aggregation propensity scores less than 35 were selected for expression and experimental assays.
N-linked glycan verification of epitope
To verify the epitope of the designed binders, an N-linked glycan scan was performed. This was performed to rapidly determine if the computational designed binder was interacting with the chosen interface50. Four engineered N-linked glycan variants (NN-0975, NN-0979, NN-0981 and NN-0977) with mutation close to the Sort_EndoTag-binding site were designed and expressed. For design, the computational models were used as a starting point for the computational screen. All positions 10âà away from the interface were screened using RosettaMatch51 followed by a design step to introduce the NXS/T motif into the protein. Computational models were minimized and filtered based on geometrical restraints, CST-score <5. Next, the four variants were used as bait in the yeast display assay against the computational designed binder, Sort_EndoTag, which was displayed on the surface of yeast.
Yeast surface display screening with FACS
The yeast surface display screening was performed as described15,17. In brief, DNAs encoding the minibinder sequences were transformed into EBY-100 yeast strain. The yeast cells were grown in CTUG medium and induced in SGCAA medium. After washing with PBSF (PBSâ+â1% BSA), the cells were incubated with 1âμM biotinylated target proteins (IGF2R, ASGPR or sortilin) together with Streptavidinâphycoerythrin (SAPE, Thermo Fisher, 1:100) and anti-Myc fluorescein isothiocyanate (FITC, Miltenyi Biotech, 6.8:100) for 30âmin. After washing twice with PBSF, the yeast cells were then resuspended in PBSF and screened via FACS. Only cells with PE and FITC double-positive signals were sorted for next-round screening. After another round of enrichment, the cells were titrated with biotinylated target protein at different concentrations for 30âmin, washed, and further stained with both Streptavidinâphycoerythrin (SAPE, Thermo Fisher) and anti-Myc fluorescein isothiocyanate (FITC, Miltenyi Biotech) at 1:100 ratio for 30âmin. After washing twice with PBSF, the yeast cells at different concentrations were sorted individually via FACS and regrown for 2 days. Next the cells from each subpool were lysated and their sequences were determined next-generation sequencing or MiSeq. FACS data were collected with the Sony SH800 software suite.
For N-linked glycan verification, yeast cells displaying Sort_EndoTag were incubated with 100nM N-glycan variants of sortilin (NN-0975, NN-0979, NN-0981 and NN-0977), separately. The percentage of yeast cells located within the pre-set gate was calculated for each N-glycan variants group and compared with the wild-type sortilin group.
Biolayer interferometry
The binding affinity for the minibinders were determined using an Octet RED96 (ForteBio). To measure the binding affinity, Streptavidin-coated biosensors (ForteBio) were first loaded with biotinylated target proteins at 50â100ânM concentration, washed with Octet buffer (10âmM HEPES, 150âmM NaCl, 3âmM EDTA, 0.05% surfactant P20 and 1% BSA), and incubated with titrated concentrations of corresponding binders. To measure the off rate (Koff), the biosensors were then dipped back into the Octet buffer. The on rate (Kon), Koff and Kd were further estimated with the Octet Analysis software.
Protein production and purification
Minibinders and minibinder fusions were expressed in E. coli BL21 as previously described1. In brief, the DNA fragments encoding the design sequences were assembled into PET-29 vectors via Gibson assembly and further transformed into BL21 strain with heat-shock. Protein expression was induced by the autoinduction system and proteins were purified with Immobilized metal affinity chromatography (IMAC) approach. Next the elutions were purified by FPLC SEC using Superdex 75 10/300 GL column (GE Healthcare). Protein concentrations were determined by NanoDrop (Thermo Scientific) and normalized by extinction coefficients.
AntibodyâEndoTag fusions were produced with a mammalian expression system. Light chain of CTX/ATZ antibody and heavy chain fused with EndoTag at C-terminal constructs were ordered in CMVR from Genscript. AntibodyâEndoTag fusions were then expressed via transient co-transfection of the EndoTag-heavy and light chains into Expi293F cells (Life Technologies) via PEI-MAX (Polyscience). In brief, 800âml cultures of Expi293F cells were transfected at a density of 3âÃâ106 cells per millilitre of culture using 1âμg plasmid DNA and 3âμg PEI per millilitre of culture. These cultures were grown in Expi293F expression medium (Life Technologies) at 37â°C in a humidified, 8% CO2 incubator rotating at 125ârpm.
After 6 days of expression, culture supernatants were harvested via 5âmin of centrifugation at 4,000g, 5âmin of incubation with PDADMAC solution (Sigma Aldrich) added to a final concentration of 0.0375%, followed by an additional 5âmin of centrifugation at 4,000g. Supernatants were clarified via 0.22-μm vacuum filtration and then treated to a final concentration of 50âmM Tris-HCl (pH 8) and 350âmM NaCl for IMAC. Gravity IMAC was performed by batch binding the clarified supernatants with 10âml of Ni Sepharose Excel resin (GE Healthcare). After 20â30âmin of incubation, the resin bed was washed with 10 column volumes of 20âmM Tris-HCl (pH 8), 300âmM NaCl solution. The proteins were then eluted with 3 column volumes of 20âmM Tris-HCl (pH 8), 300âmM NaCl, 300âmM imidazole solution. The batch bind process was then repeated with half the amount of resin (5âml) and the eluates from both batch binds were combined. SDSâPAGE was performed on the IMAC eluates to assess purity.
The purified antibodyâEndoTag fusions were subsequently concentrated in a 10âK MWCO Amicon Ultra centrifugal filter unit (Millipore) and polished via SEC using a Hiload 26/600 Superose 200 column (GE Healthcare) in DPBS (Gibco). The SEC fractions were re-concentrated in the same manner as before to a final concentration of 5âmgâmlâ1. Endotoxin levels were assayed via Endosafe LAL Endotoxin tests (Charles River) and analytical SEC was performed using a Superdex 200 Increase 5/150 column (GE Healthcare) to obtain a high-resolution size profile. Pre- and post-freeze stability was assessed via UV-vis spectrophotometry as well as SDSâPAGE.
Cellular uptake evaluation and receptor degradation via flow cytometry
For cellular uptake assays using suspension cell lines (K562, Jurkat), the cells were incubated with corresponding fluorescence-labelled protein constructs at 37â°C for indicated time, then spun down at 500g for 5âmin, resuspended and washed with cold PBS. After three washes, the cells were resuspended and transferred to a 96-well plate. For cellular uptake assays using adherent cell lines (U-251MG, Hep3B, HeLa and H1975), the cells were incubated with corresponding fluorescence-labelled protein constructs at 37â°C for indicated time, then washed with cold PBS for three times. The cells were then treated with 50âμl trypsin and incubated at 37â°C for 10âmin followed by adding 50âμl DMEM. The resuspended cells were then transferred to a 96-well plate followed by 2 PBS washes. Flow cytometry was then performed in Attune NxT flow cytometer (Thermo Fisher). The data were analysed in FlowJo v9 software.
For cell surface receptor-degradation experiments, the cells were first incubated with corresponding protein reagents for indicated time at 37â°C, then washed with cold PBS 3 times. For suspension cell lines, the cells were resuspended and transferred to the 96-well plate; for adherent cell lines, the cells were first treated with trypsin for 10âmin then transferred to the 96-well plate. The cells were then stained with corresponding fluorescence-labelled antibodies against the corresponding receptor for 1âh at room temperature. After washing three times with cold PBS for flow cytometry, flow cytometry was performed in Attune NxT flow cytometer (Thermo Fisher). The data were analysed in FlowJo v9 software. Representative gating strategy for flow cytometry is provided in Supplementary Figs. 1â17.
Monitoring protein degradation via western blot
Cells were cultured in T75 flasks at 37â°C in a 5% CO2 atmosphere. HEP3B (ATCC), HeLa (ATCC), and MDA-MB-231 were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin. Jurkat-CTLA4 (Promega, JA3001) and H1975 were cultured in RPMI supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin. Adherent cells were plated (100,000 cells per well in a 24-well plate) one day before the experiment, whereas suspension cells were plated on the day of the treatment. Cells were incubated with 250âµl of complete growth media with pLYTAC or untreated controls for indicated time. Cells were then washed with PBS 3 times and lysed with RIPA buffer supplemented with protease inhibitor cocktail (Roche), 0.1% Benzonase (Millipore-Sigma), and phosphatase inhibitor cocktail (Roche) on ice for 30âmin. The cells were scraped, transferred to Eppendorf tubes, and spun down at 21,000g for 15âmin at 4â°C. The supernatant was collected and the protein concentration was determined by BCA assay (Pierce). Equal amounts of lysates were loaded onto 4â12% Bis-Tris gel and separated by SDSâPAGE. Then, the gel was transferred onto a nitrocellulose membrane and stained with REVERT Total Protein Stain (LI-COR), then blocked with Odyssey Blocking Buffer (TBS) (LI-COR) for 1âh at room temperature. The membrane was incubated with primary antibodies (rabbit anti-EGFR D38B1 Cell Signaling Technologies, rabbit anti-HER2 2242 Cell Signaling Technologies, rabbit anti-PD-L1 E1L3N Cell Signaling Technologies, rabbit anti-CTAL4 E1V6T Cell Signaling Technologies, mouse anti-vinculin V284 Bio-Rad) overnight at 4â°C, washed 3 times with TBST. Subsequently, the membrane was incubated with secondary antibody (800CW goat anti-mouse or goat anti-rabbit LI-COR 926-32211) for 1âh at room temperature, and washed 3 times with TBST for visualization with an Odyssey CLx Imager (LI-COR). Image Studio (LI-COR) was used to quantify band intensities. Full scans of western blot gels are provided in Supplementary Figs. 1â16.
Fluorescence imaging
Wild-type HeLa (ATCC CCL-2) were cultured at 37â°C with 5% CO2 in flasks with Dulbeccoâs modified Eagle medium (DMEM) (Gibco) supplemented with 1 mM l-glutamine (Gibco), 4.5âgâlâ1 d-glucose (Gibco), 10% fetal bovine serum (FBS) (Hyclone) and 1% penicillin-streptomycin (PenStrep) (Gibco). To passage, cells were dissociated using 0.05% trypsin EDTA (Gibco) and split 1:5 or 1:10 into a new tissue culture-treated T75 flask (Thermo Scientific ref 156499).
For imaging 35-mm glass bottom dishes were seeded at a density of 20,000 cells per dish. A final monomeric concentration of 100ânM of ligands were incubated with cultured cells. Cells were fixed 4% paraformaldehyde, permeabilized with 100% methanol, and blocked with PBSâ+â1% BSA. Cells were immunostained with LAMP2A antibody (Abcam ab18528) followed by goat anti-rabbit IgG Alexa Fluor 488 secondary antibody (Thermo Fisher A-11034) and 4â²,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher D1306) and stored in the dark at 4â°C until imaging.
Cells were washed twice with HBSS and subsequently imaged in HBSS in the dark at 37â°C. Right before imaging, cells were incubated with 25âµM DTZ. Epifluorescence imaging was conducted on a Yokogawa CSU-X1 microscope equipped with a Hamamatsu ORCA-Fusion scientific CMOS camera and Lumencor Celesta light engine. Objectives used were: 10Ã, NA 0.45, WD 4.0âmm, 20Ã, NA 1.4, WD 0.13âmm, and 40Ã, NA 0.95, WD 0.17â0.25âmm with correction collar for cover glass thickness (0.11âmm to 0.23âmm) (Plan Apochromat Lambda). All epifluorescence experiments were subsequently analysed using NIS Elements software.
Generation of knockout lines
IGF2R-knockout HeLa cells were a generous gift form S. Banik. SORT1 and TfR KO cells were generated using Gene Knockout Kit v2 (Synthego) using the manufacturerâs protocols.
Confocal microscopy
Indicated cells were seeded in 18-well glass bottom µ-Slides (Ibidi, 81817) at a density of 15,000 cells per well. Fluorescently labelled ligands were incubated with the cultured cells for 0.25, 3, 6 or 24âh. Thirty minutes before image acquisition, cells were additionally incubated with LysoTracker (Thermo Fisher Scientific, L7528, L7526, L12492) was added for 30âmin. Fluorescently labelled anti-IGF2R (Novus Biological, NB300-514AF647) was added for 30âmin. Cells were washed 3à in PBS and immediately proceeded to imaging.
Confocal laser scanning microscopy was performed on a Nikon A1R HD25 system equipped with a LU-N4 laser unit (Lasers used: 488ânm, 561ânm, 640ânm). Data were acquired using a 20Ã, NA 0.75, WD 1.00âmm air objective (Plan Apochromat Lambda) in combination with 1 multialkaline (EM 650 LP) and 2 GaAsP detectors (DM 560 LP EM 524/42 (503-545) and DM 652 EM 600/45 (578-623)). Acquisition was controlled via NIS Elements software and data were analysed via Fiji and custom-written Python scripts.
Mass spectrometry and proteomics
Cell pellets were thawed on ice and lysed in a lysis buffer (400âμl, 1 tablet of Pierce EDTA-free Protease Inhibitor Tablets dissolved in 50âml of PBS) using a probe sonicator (3à 3 pulses). Protein concentration was adjusted to 2.0âmgâmlâ1 and the samples (100âμl, 200âμg protein) were transferred to new Eppendorf tubes (1.5âml) containing urea (48âmg per tube, final urea concentration: 8âM). DTT (5âμl, 200âmM fresh stock in H2O, final DTT concentration: 10âmM) was then added to the tubes and the samples were incubated at 65â°C for 15âmin. Following this incubation, iodoacetamide (5âμl, 400âmM fresh stock in H2O, final iodoacetamide concentration: 20âmM) was added and the samples were incubated in the dark at 37â°C with shaking for 30âmin. Ice-cold methanol (600âμl), CHCl3 (200âμl), and H2O (500âμl) were then added, and the mixture was vortexed and centrifuged (10,000g, 10âmin, 4â°C) to afford a protein disc at the interface between CHCl3 and aqueous layers. The top layer was aspirated without perturbing the disk, additional methanol (600âμl) was added, and the proteins were pelleted (10,000g, 10âmin, 4â°C) and used in the next step or stored at â80â°C overnight.
The resulting protein pellets were resuspended in EPPS buffer (160âμl, 200âmM, pH 8) using probe sonicator (3à 3 pulses). Trypsin (10âμl, 0.5âμgâμlâ1 in trypsin reconstitute buffer) and CaCl2 (1.8âμl, 100âmM in H2O) were added and the samples were incubated at 37â°C with shaking overnight.
Peptide concentration was determined using the microBCA assay (Thermo Scientific) according to the manufacturerâs instructions. For each sample, a volume corresponding to 25âμg of peptides was transferred to a new Eppendorf tube and the total volume was brought up to 35âμl with EPPS buffer (200âmM, pH 8). The samples were diluted with CH3CN (9âμl) and incubated with the corresponding TMT tags (3âμl per channel, 20âμgâμlâ1) at room temperature for 30âmin. An additional TMT tag (3âμl per channel, 20âμgâμlâ1, 30âmin) was added and the samples were incubated for another 30âmin. Labeling was quenched by the addition of hydroxylamine (6âμl, 5% in H2O). Following a 15âmin incubation at room temperature, formic acid was added (2.5âμl, final formic acid concentration: 5%). Twenty microlitres of labelled peptides for each channel were combined into a 2.0âml low-binding Eppendorf tube, and 25âμl of 20% formic acid was added. The resulting mixture was lyophilized to remove the solvents before high pH fractionation.
The spin columns from Pierce High pH Reversed-Phase Peptide Fractionation Kit were pre-equilibrated prior to use. In brief, the columns were placed in Eppendorf tubes (2âml), spun down to remove the storage solution (5,000g, 2âmin), and washed with CH3CN (2à 300âμl, 5,000g, 2âmin) and buffer A (2à 300âμl, 95% H2O, 5% CH3CN, 0.1% formic acid, 5,000g, 2âmin). TMT-labelled peptides were re-dissolved in buffer A (300âμl, 95% H2O, 5% CH3CN, 0.1% formic acid) and loaded onto pre-equilibrated spin columns for high pH fractionation. The columns were spun down (2,000g, 2âmin) and the flow through was used to wash the original Eppendorf tube and passed through the spin column again (2,000g, 2âmin). The column was then washed with buffer A (300âμl, 2,000g, 2âmin) and 10âmM aqueous NH4HCO3 containing 5% CH3CN (300âμl, 2,000g, 2âmin), and the flow through was discarded. The peptides were eluted from the spin column into fresh Eppendorf tubes (2.0âml) with a series of 10âmM NH4HCO3/CH3CN buffers (2,000g, 2âmin). The following buffers were used for peptide elution (CH3CN (%)): 7.5, 10, 12.5, 15, 17.5, 20, 22.5, 25, 27.5, 30, 32.5, 35, 37.5, 40, 42.5, 45, 47.5, 50, 52.5, 55, 57.5, 60, 62.5, 65, 67.5, 70, 72.5, 75, 80 and 95. Every tenth fraction was combined into a new clean Eppendorf tube (2âml) and the solvent was removed using a benchtop lyophilizer and stored at â20â°C before analysis.
The resulting 10 combined fractions were resuspended in buffer A (25âμl) and analysed on the Orbitrap Fusion mass-spectrometer (4âμl injection volume) coupled to a Thermo Scientific EASY-nLC 1200 LC system and autosampler. The peptides were eluted onto a capillary column (75 μm inner diameter fused silica, packed with C18) and separated at a flow rate of 0.3âμl/minâ1 using the following gradient: 5% buffer B in buffer A from 0â10âmin, 5%â35% buffer B from 10â129âmin, 35%â100% buffer B from 129â130âmin, 100% buffer B from 130â139âmin, 100%â5% buffer B from 139â140âmin, and 5% buffer B from 140â150âmin (buffer A: 100% H2O, 0.1% formic acid; buffer B: 20% H2O, 80% CH3CN, 0.1% formic acid). Data were acquired using an MS3-based TMT method. In brief, the scan sequence began with an MS1 master scan (Orbitrap analysis, resolution 120,000, 375âââ1,600âm/z, cycle time 3âs) with dynamic exclusion enabled (repeat count 1, duration 30âs). The top precursors were then selected for MS2/MS3 analysis. MS2 analysis consisted of: quadrupole isolation (isolation window was set to 1.2 for charge state zâ=â2; 0.7 for charge state zâ=â3; 0.5 for charge states zâ=â4â6) of precursor ion followed by collision-induced dissociation (CID) in the ion trap (normalized collision energy 35%, maximum injection time 50âms, MS2 resolution was set to turbo). Following the acquisition of each MS2 spectrum, synchronous precursor selection (SPS) enabled the selection of MS2 fragment ions for MS3 analysis (SPS isolation window was set to 1.3 for charge state zâ=â2; 0.7 for charge state zâ=â3; 0.5 for charge states zâ=â4â6). MS3 precursors were fragmented by HCD and analysed using the Orbitrap (collision energy 65%, maximum injection time 120âms). The raw files were converted to mzML files using the MSConvert tool from ProteoWizard (version 3.0.22088). A reverse concatenated, non-redundant variant of the Human UniProt database (29 November 2022) was searched using FragPipe (version 18.0) with the built-in TMT10-MS3 workflow. The virtual references were used for the data sets due to the lack of a pooled sample. The quantified proteins were filtered with false discovery rateâ<â1% with median centreing normalization. Data are presented as the mean fold change to DMSO-treated controls. nâ=â3 per group. P values were calculated by a two-tailed unpaired t-test with Welchâs correction.
IgG and LHDB supernatant clearance assays
Jurkat or K562 cells seeded in 96-well culture plates in 300âμl medium were incubated with AF647-conjugated IgG (Novusbio) or LHDB alone or together with protein G-EndoTag reagents. At various timepoints, the cells were pelleted down and 30âμl of supernatants were extracted and further diluted to 45âμl by using a PBS buffer. After shaking in an orbital shaker for 5âmin, the fluorescence intensity was measured using a Neo2 plate reader (BioTek) at wavelength 647ânm. The percentage clearance was measured by normalizing the control group without adding protein G-EndoTag reagent.
SEC binding assay
ASGPR protein (28.4âkDa) at 1âμM (diluted in PBS) was incubated with 3âμM AS_EndoTag-3C for 30âmin and run through an ÃKTA SEC protein purification system using a S200 16/90 column. The absorbance at 230ânm was used as a readout for binding. The SEC traces of the complex was compared to the traces of individual ASGPR or AS_EndoTag-3C at same concentration.
In vivo mouse study
Mouse lymphoma cell line A20 cell was purchased from American Type Culture Collection (ATCC, TIB-208). The cell was cultured in RPMI-1640 medium (Gibco, Thermo Scientific, 31870074), supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Thermo Scientific), 1à GlutaMAX (Gibco, Thermo Scientific), 1à penicillin/streptomycin solution (Gibco, Thermo Scientific). The cell was cultured at 37â°C in humidified condition with 5% CO2.
All animal experiments were conducted at the Instituto de Medicina Molecular João Lobo Antunes (IMM), Lisbon. Animal work was performed in strict accordance with Portuguese Law (Portaria 1005/92) and the European Guideline 86/609/EEC and follow the Federation of European Laboratory Animal Science Associations guidelines and recommendations concerning laboratory animal welfare. All animal experiments were approved by the Portuguese official veterinary department for welfare licensing (Direção Geral de Alimentação e Veterinária) and the IMM Animal Ethics Committee (authorization AWB_2021_03_GB_Targ CancerDrugs). Eight-week-old female BALB/c mice (purchased from Charles River) were used in this study, with 5âÃâ106 A20 cells inoculated subcutaneously in the flank. Tumour growth was monitored over time, by performing bilateral vernier caliper measurements every day and mean tumour volumes were calculated using the formula (lengthâÃâwidth2)/2. Treatments were initiated when tumours reached approximately 100 mm3 (approximately 10 days after tumour induction), with the mice been randomly assigned to receive ATZ, ATZâIGF_EndoTag1 or ATZâIGF_EndoTag4 and isotype as controls (nâ=â6 mice per group). Treatments were administered intratumourally in a total of three injections for every three days. Animals were monitored every day; tumours were measured as described before and mouse weight was evaluated throughout the study. Animals were killed whenever reaching humane endpoints: loss of 20% of body weight, breathing impairment, or poor reaction to external stimuli. No signs of animal suffering or discomfort were observed during the experiment. For efficacy study, once control (isotype-treated mice) tumours reached 1,000âmm3, all mice were killed (by isoflurane overdose), and the tumours were removed for western blot analysis. For survival monitor, each mouse was killed respectively when tumours reached 1,000 mm3. The light/dark cycle was 14âh light/10âh dark (lights on at 07:00; lights off at 21:00). The temperature was 20â24â°C and the relative humidity was 55â±â10%, with controlled supply of HEPA-filtered air provided to individually ventilated cages. Maximum number of animals per cage was five. Social isolation was avoided whenever possible. The type of food was autoclaved diet pellets RM3A (P), from SDS Special Diets Services (801030). Food was placed in a grid inside the cage and provided ad libitum to animals. The type of water was sterile water treated by reverse osmosis. Water was provided ad libitum to animals through bottles with a capillary hole. The data collected was analysed using GraphPad Prism9.
In vivo PD-L1 degradation of tumour samples by western blot
Tumour samples isolated from the mice were homogenized, lysed in RIPA buffer containing protease inhibitor (Roche), phosphatase inhibitors (Sigma) and 0.1% Benzonase (Sigma) on ice for 30âmin. The lysates were spun at 21,000g for 15âmin at 4â°C. The supernatant was collected, and the protein concentrations were quantified using BCA assay (Sigma). Fifty micrograms of protein were loaded per lane and separated on 12% SDSâPAGE gels, and then transferred onto polyvinylidene difluoride (PVDF) membranes (GE Healthcare). Membranes were then blocked with 5% BSA in TBS supplemented with 0.1% Tween-20 (TBST) for 1âh at room temperature, and then probed with following specific primary antibodies at 4â°C overnight. After three times of washing with TBST, secondary antibodies were added to the membrane for 1âh at room temperature. All membranes were washed three times and exposed using ECL substrate (Bio-Rad, 170â5060) and Amersham 800 Imaging System (Cytiva). The primary antibodies used included PD-L1 (sc-518027) and beta-actin (sc-47778), the secondary antibody was goat anti-mouse IgG H&L (HRP) (Abcam, ab205719). The intensities of the bands were quantified by ImageJ.
Statistical analysis
No statistical analysis was used to determine the sample size. The sample size was determined by our ability to detect meaningful differences between treatments. Western blot experiments in vitro and BLI binding assays were conducted with sample size of one based on low variance from previous experience. Western blot experiments in mice were performed with sample size of 3 to reduce the variance of protein level across animals from our previous best practice. The data were collected as biological replicates as indicated in the figure legend. All cell experiments were done multiple times to ensure reproducibility. All images were representative of three independently replicated samples. Statistical analyses are specified in figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.