Animals
Experiments were performed in accordance with ethics guidelines approved by The University of Melbourne, Monash University and St Vincents Research Institutes Animal Ethics Committee (10323, 10324, 10352, 10385, 10427, 21712, 22282, 22404, 25349 and 28097). Mice were maintained on a 12âh light-dark cycle in a temperature-controlled high-barrier facility with free access to food and water per NHMRC Australian Code of Practice for the Care and Use of Animals. C57BL/6J mice were sourced from the Animal Resources Centre, Australia, whereas Agrp-IRES-Cre (strain 012899), db/db (strain 000697), Npy-GFP (strain 006417), Pomc-GFP (strain 009593), LSL-Cas9 (strain 028551), NZO (strain 002105) mice were sourced from Jackson Laboratories. To generate Agrp-IRES-Cre;;LSL-Cas9-GFP (AgRP-Cas9) mice, hemizygous Agrp-IRES-Cre mice were bred with homozygous LSL-Cas9-GFP mice. All experimental interventions were performed in male rodents aged 8â10 weeks old, unless stated otherwise. Male SpragueâDawley rats (Animal Resources Centre, Australia) were housed individually with nesting and enrichment material at a room temperature of 23â±â2â°C, room humidity 40â70%, on a reverse 12âh light/dark cycle (lights off at 09:00). Animals were fed a standard chow (Barastoc, Ridley AgriProducts), a high-fat high-sugar diet (mice: 43% and 20% of total energy from fat and carbohydrate respectively, SF04-001, Specialty Feeds; rats: 30% fats of total energy SF17-204, Specialty Feeds) or a high-fat high-cholesterol diet (40% of total energy from fat and 2% cholesterol, SF16-033, Specialty Feeds). To induce late-stage type 2 diabetes in mice, male C57Bl/6J mice were fed a HFHS diet for 4 weeks before being receiving up to 6 intraperitoneal injections of streptozotocin (40âmgâkgâ1 (Sigma) in 50âmM sodium citrate buffer pH 4.5) over the following 2 weeks. Blood glucose levels were monitored and mice exhibiting stable blood glucose levels of >15âmM were used for downstream experiments. For all experiments, random allocation was used for assignment of individual mice to experimental groups, and sample sizes were chosen on the basis of prior work and according to standards in the field.Â
Genotyping
DNA was extracted from tail biopsies using Tissue Extract-PCR Buffers (MDX004, Meridian Bioscience) and DNA was amplified by PCR using MyTaq HS Red Mix (BIO-25048, Meridian Bioscience) with the following primers to detect cre (forward: 5â²-GCGGTCTGGCAGTAAAAACTATC-3â², reverse: 5â²-GTGAAACAGCATTGCTGTCACTT-3â²), LSL-Cas9 (wt forward: 5â²-AAGGGAGCTGCAGTGGAGTA-3â², wt reverse: 5â²-mCAGGACAACGCCCACACA-3â², mt forward: 5â²-TCCCCATCAAGCTGATCC -3â², mt reverse: 5â²-CTTCTTCTTTGGGGCCATCT-3â²), Npy-GFP (common forward: 5â²-TATGTGGACGGGGCAGAAGATCCAGG-3â², wt reverse: 5â²-CCCAGCTCACATATTTATCTAGAG-3â², mt reverse: 5â²-GGTGCGGTTGCCGTACTGGA-3â²), Pomc-GFP (forward 5â²-AAGTTCATCTGCACCACCG-3â², reverse 5â²-TGCTCAGGTAGTGGTTGTCG-3â²) alleles. The following primers were used to monitor the CRISPR-mediated deletion of the mouse insulin receptor gene (ÎInsrCRISPR): forward 5â²-GAGATGGTCCACCTGAAGGA-3â², reverse 5â²-GTGAAGGTCTTGGCAGAAGC-3â².
Immunohistochemistry
For immunohistochemistry on brain, mice were anaesthetized and perfused transcardially with heparinized saline (10,000âunitsâlâ1 porcine heparin) followed by 10% neutral buffered formalin. Brains were post-fixed for 16âh and kept for three days at 4â°C in 30% sucrose in PBS to cryoprotect the tissue, before freezing on dry ice. Thirty-micrometre sections (120âmm apart) were cut in the coronal plane throughout the entire rostralâcaudal extent of the hypothalamus. Sections were stored in cryoprotectant (30% ethylene glycol, 20% glycerol in PBS) at â20â°C for long term storage. For the detection of hyaluronic acid and versican only, sections were subjected to heat-induced epitope retrieval using citrate acid buffer (10âmM sodium citrate, 0.05% Tween 20, pH 6.0) at 95â°C for 20âmin.
For detection of aggrecan, GFP, hyaluronic acid, mCherry, versican, tenascin C, HAPLN1, neurocan, phosphacan, brevican, WFA, WFAâFITC, PGP9.5 and AgRP, sections were incubated at room temperature for 1âh in blocking buffer (0.3% Triton X-100, 5% normal goat serum, Gibco, Thermo Fisher, 0.02% sodium azide) and then overnight at 4â°C in 1% blocking buffer containing either rabbit anti-aggrecan (1:1,000, AB1031, Millipore), chicken anti-GFP (1:2,000; ab13970, Abcam), biotinylated hyaluronic acid binding protein (1:100, 385911, Millipore), rabbit anti-dsRed (1:2,000, 600-401-379, Rockland), rabbit anti-versican (1:1,000, AB1033, Millipore), tenascin C (1:500, M1-B4, Developmental Studies Hybridoma Bank), HAPLN1 (1:500, 9/30/8-A-4, Developmental Studies Hybridoma Bank), neurocan (1:300, 1F6-S, Developmental Studies Hybridoma Bank), phosphacan (1:300, 3F8, Developmental Studies Hybridoma Bank), brevican (1:500, 610895, BD Transduction Laboratories), biotinylated WFA (1:2,000, L1516; Sigma-Aldrich), WFAâFITC (1:2,000, FL-1351-2, Vector Laboratories), rabbit anti PGP9.5 (1:1,000, 14730-1-AP, Proteintech), or guinea pig anti-AgRP (1:500, AS506, Antibodies Australia). After washing with PBS-T (0.3% Triton X-100 in PBSâ+â0.02% sodium azide), sections were incubated with goat anti-chickenâAlexa Fluor 488 (ab150169, Abcam), goat anti-rabbitâAlexa Fluor 488, goat anti-rabbitâAlexa Fluor 595 or goat anti-rabbitâAlexa Fluor 647 (ab150077, ab150080 or ab150083, respectively, Abcam), StreptavidinâAlexa Fluor 594 or StreptavidinâAlexa Fluor 647 Streptavidin (405240, BioLegend) in 5% blocking buffer for 2âh at room temperature. Sections were mounted with Mowiol 4â88 mounting media and visualized with an Olympus BX61 microscope. Images were captured with an Olympus BX61 camera, acquired using Olympus cellSens Dimension software v2.1 and processed using ImageJ software v1.53âs (NIH). Images for cell internalization were captured using a Zeiss LSM880 Airyscan Fast confocal microscope, acquired using Zeiss ZEN software v2.1 and processed using ImageJ v1.53âs (NIH). Brightness and contrast have been adjusted to aid in the analysis and visualization.
For ingWAT, eWAT, liver and BAT immunohistochemistry, tissue was immediately dissected and fixed in buffered formalin solution on a rocking platform for 48âh at room temperature. Tissues were embedded in paraffin, and 5-µm sections 100âµm apart were prepared. For haematoxylin and eosin histology, sections were incubated in haematoxylin for 3âmin followed by 30âs in eosin. For detection of UCP1, ingWAT sections were subjected to antigen retrieval in citrate acid buffer (10âmM sodium citrate, 0.05% Tween 20, pH 6.0) at 95â°C for 20âmin. Sections were incubated at room temperature for 1âh in 5% blocking buffer and then overnight at 4â°C in rabbit anti-UCP1 (1:1,000; ab10983, Abcam), in 1% blocking buffer. Following washing in PBS-T, sections were incubated with goat anti-rabbit Alexa Fluor 488 (ab150077, Abcam) secondary antibody in 5% blocking buffer for 2âh at room temperature. Sections were incubated in DAPI (20ângâmlâ1 in PBS) for 10âmin then mounted with Mowiol 4â88 mounting media and visualized with an Olympus BX61 microscope. Images were captured with an Olympus BX61 camera, acquired using Olympus cellSens Dimension software v2.1 and processed using ImageJ software v1.53âs (NIH). Brightness and contrast have been adjusted to aid in the analysis and visualization.
Functional p-AKT and p-STAT3 Immunohistochemistry
Mice were injected intraperitoneally with vehicle (PBS) or insulin (3âmUâgâ1, Actrapid, Nova Nordisk) and mice were transcardially perfused (as described above) after 15âmin with 10% neutral buffered formalin. For p-STAT3 signalling, mice were intravenously injected with vehicle (PBS) or leptin (20âμg per mouse in a volume of 100âμl) and transcardially perfused after 30âmin with 10% neutral buffered formalin. The brains were post-fixed for 16âh on a rocking platform at room temperature and then kept for two days in 30% sucrose in PBS to cryoprotect the tissue, before freezing on dry ice. 30âµm sections were cut in the coronal plane throughout the entire rostralâcaudal extent of the hypothalamus. Sections were pre-treated for 20âmin in freshly prepared 1% NaOH, 1% H2O2 in PBS, washed in PBS, incubated for 10âmin in 0.3% glycine, washed in PBS and incubated for 10âmin in 0.03% SDS. Sections were then blocked in 5% blocking buffer for 1âh at room temperature and incubated for 48âh with rabbit anti-p-AKT (Ser473) (1:300; 4060, Cell Signaling Technology), rabbit anti p-STAT3 (Tyr705) (1:500, number 9131S, Cell Signaling Technology), or rabbit anti fluor in 1% blocking buffer. Sections were then incubated in 5% blocking buffer containing either goat anti-rabbit Alexa Fluor 647 (ab150083, Abcam), goat anti-rabbit Alexa Fluor 594 (ab150080, Abcam) or biotinylated goat anti-rabbit (BA-1000, Vector Laboratories, no sodium azide in 5% blocking buffer). Florescence sections were mounted with Mowiol 4â88 mounting media and visualized using Olympus BX61 microscope. Images were captured with an Olympus BX61 camera, acquired using Olympus cellSens Dimension software v2.1 and processed using ImageJ software v1.53âs (NIH). For chromogenic detection, p-AKT signal was amplified using Vectastain ABC-HRP Kit (1;500, PK-4000, Vector Laboratories) and visualized using 0.1% H2O2 DAB solution (3,30-diaminobenzidine, ICN980681, Thermo Fisher) Peroxidase Substrate Kits (Vector Laboratories). p-STAT3 and p-AKT immunopositive cells were visualized with a Leica DM2000 LED bright field microscope using a Leica DMC6200 camera and Leica Application Suite X software. Brightness and contrast have been adjusted to aid in the analysis and visualization.
PNN immunofluorescent analysis
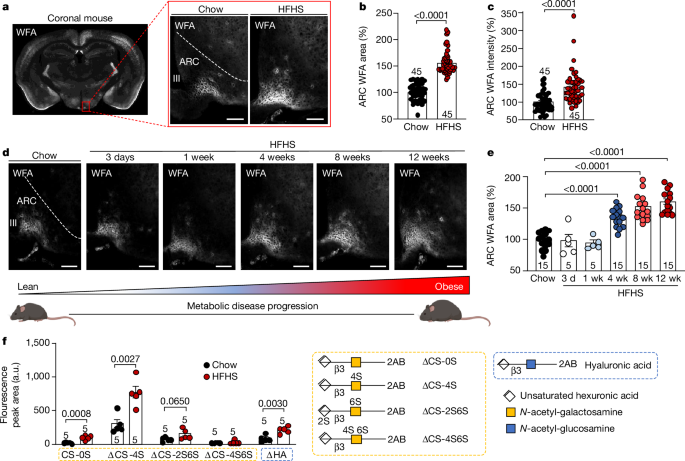
The ARC PNN was stereologically assessed throughout the entire rostro-caudal ARC. The ARC was divided into three regions, including the rostral ARC (â1.22/â1.58âmm anteriorâposterior), medial ARC (â1.58/â1.94âmm anteriorâposterior) and caudal ARC (â1.94/â2.18âmm anteriorâposterior). PNN was quantified in the VMH and RSG cortex (â1.58/â1.94âmm anteriorâposterior).
All image quantification was performed in ImageJ v1.53âs (NIH). Raw images underwent background subtraction using a rolling ball algorithm to minimize background and any potential variance in tissue autofluorescence. To quantify area and intensity of the PNN within each brain region (ARC, VMH or RSG cortex) images were thresholded and binarized to create a region of interest (ROI) mask of only the PNN. For each brain area, PNN ROI area (µm2) and intensity was calculated. This process was automated to minimize bias and to account for differences in brain nuclei size across multiple images. Brain nuclei were defined in accordance with the Paxinos and Franklin Mouse Brain Atlas (http://labs.gaidi.ca/mouse-brain-atlas/). The area and intensity of PNN within each region was normalized to the respective control.
To determine the co-localization of ECM components (hyaluronic acid, HAPLN1, tenascin C, aggrecan, versican, phosphacan, brevican, neurocan) within the PNN (WFA-positive staining), 2 masks were generated per image: one for the total PNN staining and another for component staining within the ARC. The overall area and intensity were calculated for the total PNN structure. The area and intensity for components within the PNN was determined by quantifying the expression within the total PNN mask only. This allowed for the characterization of ECM components expressed specifically within the ARC PNN. The area and intensity of PNN within each region was normalized to the respective control. To determine the co-localization of the WFA-labelled ARC PNN within the ARC PNN components, two masks were generated per image: one for total PNN staining and another for component staining within the ARC. The overall area and intensity were calculated for the total component structure. The area and intensity for the PNN comprising the components was determined by quantifying the WFA expression within the total component mask only. The area and intensity of PNN within each region was normalized to the respective control. This combined approach further characterizes the specificity of the components to the PNN region. Brightness and contrast have been adjusted to aid in the analysis and visualization.
Quantification of ARC neurons within the PNN
To determine which metabolically relevant ARC neurons are encased within the PNN during the development of metabolic disease we analysed brains taken from 0, 4- and 12-week HFHS-fed Npy-GFP (to visualize AgRP/NPY neurons) and Pomc-GFP (to visualize POMC neurons) mice. ARC sections were stained for GFP and WFA as described in âImmunohistochemistryâ and analysed using ImageJ v1.53âs (NIH) software. To determine the number of GFP positive neurons encased within the PNN we generated two masks. To define the PNN structure in the ARC, images were thresholded and binarized to create a PNN mask. To identify individual GFP positive neurons, images were thresholded and binarized to create a GFP mask. To define individual GFP neurons, the GFP masks were segmented using a watershed separation algorithm. The total number of GFP positive cells were counted within the whole ARC area and within the PNN mask. This quantified the percent of GFP cells encompassed by the PNN in the ARC.
To determine the intensity of the PNN that specifically surrounds individual GFP cells in the ARC, GFP images were thresholded and binarized. An ROI of 1.29âµm (average size of ECM surrounding cortical neurons47) was generated around each GFP cell using dilate, distance map and Voronoi processes in ImageJ v1.53âs software. This generated a mask capable of specifically analysing PNN bordering individual GFP cells. Using this mask, PNN staining intensity surrounding GFP cells present within the ARC PNN was determined.
Behavioural satiety sequence
Mice were fasted overnight and housed individually in transparent cages with ad libitum access to water. Two hours after the beginning of the light cycle (at 09:00) pre-weighed food was presented to the mice and mice were undisturbed and discreetly observed for 90âmin. Momentary behaviour was scored every 30âs over a 90-min observation. Behaviour at each 30âs interval was recorded according to the following classifications: feeding (animal at hopper trying to obtain food, chewing, or gnawing), drinking (animal licking at the water spout), grooming (animal scratching, biting or licking any part of its anatomy), resting (animal curled up, resting head with eyes closed), active (animal showing activity, including locomotion, sniffing, rearing), or inactive (animal immobile when aware, or signs of sickness behaviour). Data were collated into 5-min bins, and several variables were assessed including the average percentage of time the mice spent engaging in each recorded behaviour (percentage of total behaviour), food intake, the transition from eating to resting and the time to satiety (the time when the frequency of eating behaviour intersects with the frequency of resting behaviour).
Stereotaxic surgery
All stereotaxic injections were undertaken under isoflurane anaesthetic using an Ultra Precise Stereotaxic Instrument (963 Kopf) or Ultra Precise Rotational Stereotaxic Instrument (69100, RWD Life Sciences) alongside stereotaxic nanoinjectors (788130, KD Scientific) with Neurosyringes (Hamilton). To induce hypothalamic inflammation, mice were bilaterally injected with a 1:1:1 cocktail containing AAVs expressing GFP (AAV-CMV-eGFP, Addgene) and ligands for TNF (AAV-CMV-TNF) and TGFβ (AAV-CMV-TGFβ) or control AAV alone (AAV-CMV-eGFP, Addgene). To inhibit hypothalamic inflammation mice received bilateral injections of a 1:1:1:1 AAV cocktail containing expressing soluble TNF Receptor Superfamily 1âA (AAV-CMV-sTNFR1A), soluble TGFβR2 (AAV-TRE-sTGFβR2 and AAV-CMV-TetOFF) and AAV-CMV-eGFP vector, or control AAV alone (AAV-CMV-eGFP, Addgene). All inflammatory AAVs we delivered at ~1012âGUâmlâ1. To disassemble the PNN within the ARC, mice received bilateral (unless stated otherwise) administration of 15âmU per side of active chABC (C3667, Sigma; dissolved in 1âM trehalose) or heat-inactivated chABC protein as a vehicle (chABC in 1âM trehalose48 was heat-inactivated at 85â°C for 45âmin, as previously described49) in a total volume of 150ânl per side. To pulse the PNN within the ARC or RSG, mice received bilateral (unless stated otherwise) administration of biotinylated WFA (0.3âµg per side, in a volume of 150ânl). To disrupt the insulin receptor in AgRP neurons, 12-week HFHS-fed AgRP-Cas9 mice were stereotaxically injected with AAV vectors expressing U6-driven guide RNAâs targeting the Insr gene or a scrambled sequence (5â²-GTGTAGTTCGACCATTCGTG-3â²) alongside a CAG driven mCherry FLEX switch. Unless otherwise stated injections were delivered bilaterally into the ARC (coordinates, bregma: anteriorâposterior, â1.70âmm; dorsalâventral, â5.85âmm; lateral, ±0.18âmm, 200ânl per side) or into the RSG (coordinates, bregma: anteriorâposterior, â1.70âmm; dorsalâventral, â1.00âmm; lateral, ±0.20âmm, 200ânl per side). WFAâbiotin was injected unilaterally into the cc (coordinates, bregma: anteriorâposterior, â1.70âmm; dorsalâventral, â1.50âmm; lateral, ±0.20âmm, 200ânl per side).
Hyperinsulinaemicâeuglycaemic clamps in conscious freely behaving mice
For hyperinsulinaemicâeuglycaemic clamps, mice were anaesthetized under isoflurane and the right jugular vein was catheterized for infusions, as previously described4. Catheters were attached to an implant button (BMSW25, RWD Life Sciences). Implant buttons were capped allowing for group mousing of mice and catheters were kept patent by flushing daily with 40âµl heparinized saline. On the day of the experiment, food was removed at 07:00. After 3.5âh fasting, a primed (1âmin, 1.25âμCiâminâ1) continuous infusion (0.05âμCiâminâ1) of [3-3H]glucose (NET331A001MC, PerkinElmer) was administered to measure whole-body glucose turnover, as described4. Ninety minutes later, mice received a 40âmUâkgâ1 insulin bolus over 10âmin which was followed by continuous insulin infusion (4âmUâkgâ1âminâ1 in gelofusine). Euglycaemia (~8â10âmM blood glucose) was maintained by a variable infusion of a 30% glucose solution.
Tail blood samples were collected during steady-state conditions (rate of appearance (Ra) = rate of disappearance (Rd)) and at 80, 90, 100, 110, and 120âmin for determination of Rd and Ra, as described above. At 120âmin, a 13âμCiâ bolus of [14C]-2-deoxy-d-glucose (NEC495A250UC, PerkinElmer) was injected into the jugular vein, and blood was sampled at 122, 125, 135, 145 and 155âmin. At the end of the experiment tissues were extracted for glucose uptake determinations.
Pair feeding
HFHS-fed C57BL/6J mice were bilaterally injected with vehicle or chABC into the ARC. 24âh food intake was determined for intra-ARC chABC-treated mice and a cohort of intra-ARC treated vehicle-treated mice were pair-fed, whereby food availability was restricted to the average food consumed by intra-ARC chABC-treated mice.
Metabolic assessment
Metabolic measurements were undertaken in the Melbourne Mouse Metabolic Phenotyping Platform (The University of Melbourne, Australia). Glucose tolerance tests were performed on 6âh fasted conscious mice respectively by injecting d-glucose (2âmg per g of lean body mass and 1âmg per g lean mass for db/db and HFHSâ+âstreptozotocin mice) into the peritoneal cavity and measuring glucose in tail blood immediately before and at 0, 15, 30, 45, 60, 90 and 120âmin after injection using an Accu-Check glucometer (Roche). The areas under glucose excursion curves were determined and expressed as mMâÃâmin. Fasted (12âh fast) plasma insulin or glucose levels were determined using a Rat/Mouse Insulin ELISA (EZRMI-13K, Merck Millipore) or an Accu-Check glucometer respectively. The HOMA-IR was calculated using the equation [(glucoseâÃâinsulin)/405]. Adiposity was measured using TD-NMR minispec with OPUS 7.0 spectroscopy software (Bruker Optics).
Mice were acclimated for 24âh and then monitored for 48âh in an environmentally controlled Promethion Metabolic Screening System (Sable Systems International) fitted with indirect open circuit calorimetry, food consumption and activity monitors to measure activity, caloric intake and energy expenditure. Data were recorded and extracted using MetaScreen v2.3.15.13 and Macro Interpreter v23.6.0 (Sable Systems International). Respiratory quotients were calculated as the ratio of CO2 production over O2 consumption respiratory exchange ratio and energy expenditure was calculated using the Weir equation (energy expenditure (kcalâhâ1)â=â60âÃâ(0.003941âÃâVO2â+â0.001106âÃâVCO2). To account for difference in body mass/composition energy expenditure was analysed and adjusted using ANCOVA using scripts available at the National Mouse Metabolic Phenotyping Centers (MMPC) energy expenditure analysis page (https://www.mmpc.org/shared/regression.aspx).
To provide an index of ingWAT and BAT thermogenesis, infrared thermography was used to measure temperature changes in the inguinal and interscapular regions as described previously50. The FLIR T1010 thermal imaging camera (FLIR Systems Australia) was mounted onto a tripod and animals were positioned at a standardized distance of 70âcm from the camera. Animals were anaesthetized, shaved in the regions of interest and whole-body images were collected in both the prone and supine positions. Temperatures were analysed using the FLIR ResearchIT Max 4 program (FLIR Systems). The peak temperatures within the ingWAT and BAT was determined.
Viral vector production
To generate the AAV-gScrambled (pAAV-U6>mScramble-GTGTAGTTCGACCATTCGTG)-CAGâ>âLL:rev(mCherry):rev(LL):WPRE) and AAV-gIR (pAAV[-U6>mInsr[gRNA-TATCGACTGGTCCCGTATCC]-U6>mInsr[gRNA-GTCTGTCCAGGCACCGCCAA]-CAGâ>âLL:rev(mCherry):rev(LL):WPRE) viral vectors, sgRNAs were first designed using online CRISPR tools (http://crispr.mit.edu and http://chopchop.cbu.uib.no/). Potential off-target gRNA binding was assessed in silico using Off-Spotter (https://cm.jefferson.edu/Off-Spotter/) and guides exhibiting â¥3 mismatch with non-specific genomic regions were considered. For AAV-gScrambled a pUp-U6>Scrambled gRNA vector was generated using the Gibson assembly of a pDONR P4-P1R backbone and primers 5â²-GGGGACAACTTTGTATAGAAAAGTTGGAGGGCCTATTTCCCATGATTC-3â² and 5â²-GGGGACTGCTTTTTTGTACAAACTTGAAAAAAGCACCGACTCGGTGCC-3â². For AAV-gIR a pUp-U6>mInsr[gRNA-TATCGACTGGTCCCGTATCC]-U6>mInsr[gRNA-GTCTGTCCAGGCACCGCCAA] gRNA vector was generated using the Gibson assembly of a AarI digested pUp-U6-gRNA-AarI-Stuffer-AarI backbone and primers 5â²-ATATCTTGTGGAAAGGACGAAACACCGTATCGACTGGTCCCGTATCCG-3â² and 5â²-AACTTGCTATTTCTAGCTCTAAAACTTGGCGGTGCCTGGACAGAC-3â². For both AAV-gScrambled and AAV-gIR the p-Up vectors were cloned alongside pDown-CAG and pTail-LL:rev(mCherry):rev(LL) to generate the final vectors by LR reaction using the Gateway method. The AAV plasmids were used to generate recombinant viral vectors packaged into the AAV-DJ/8 pseudotype supplied at a titre of >2âÃâ1013âGCâmlâ1). All vector cloning and AAV packaging was carried out by VectorBuilder (Chicago, IL). The recombinant AAV vectors expressing inflammatory factors TNF (AAV-CMV-TNF) and TGFβ (AAV-CMV-TGFβ), or soluble TNF receptor superfamily 1A (AAV-CMV-sTNFRA1) or TGFβ receptor 2 (AAV-TRE-sTGFβR2 and AAV-CMV-TetOFF), were manufactured in-house as described previously51. In brief, cDNA constructs carrying the relevant gene expression cassettes flanked by AV2 terminal repeats in an AAV expression plasmid were transfected with the pDGM6 packaging plasmid into HEK293T cells (Sigma; authenticated by Sigma and not tested for mycoplasma contamination) by means of the calcium phosphate precipitate method to produce AAV6 vectors. At 72âh after transfection, the media and cells were harvested for purification via heparin affinity column (HiTrap, GE Healthcare) chromatography and overnight ultracentrifugation before re-suspension in sterile physiological Ringerâs solution and titre determination via quantitative PCR-based reaction (Applied Biosystems) as described previously52. Purified vectors were stored frozen until the day of use, at which time they were rapidly thawed at room temperature and diluted in sterile PBS for administration via stereotaxic injection as described herein.
Insulin and leptin extravasation in the ARC
12-week HFHS-fed C57BL/6J or aged-matched chow-fed controls received bilateral injections of vehicle or chABC into the ARC. 3 days post-injection (before differences in body weights were seen), mice were fasted for 6âh. To assess insulin extravasation into the ARC mice were administered insulinâFITC (50âµg per mouse in a volume of 100âµl, intravenous injection, I3661, Sigma) or FITC (64.3âµmol per mouse in a volume of 100âµl, intravenous injection, F3651, Sigma). To assess leptin extravasation into the ARC, mice were administered leptin-647 (20âμg per mouse in a volume of 100âμl). Mice were perfused (as described above) 30âmin post-injection. To assess insulin extravasation into the ARC irrespective of the BBB, mice were administered insulinâFITC (1âµg per mouse in a volume of 2âµl) directly into the lateral ventricles. To do this, mice were anaesthetized and stereotaxically injected (as described above) insulinâFITC at a rate of 200ânlâminâ1 into the lateral ventricles (coordinates, bregma: anteriorâposterior, â0.20âmm; dorsalâventral, â2.4âmm; lateral, +0.10âmm). Mice were perfused (as described above) 20âmin from the start of injection. To assess insulinâFITC brains were post-fixed overnight and cryoprotected in 30% sucrose in PBS. To retain spontaneous fluorescence signal, brains and sections were kept in the dark and were mounted and imaged immediately after sectioning.
Lateral ventricle cannulations
Under isoflurane anaesthetic 12-week HFHS-fed C57BL/6J or AgRP-Cas9 mice were implanted stereotaxically with guide cannulas into the right lateral ventricle (0.2âmm posterior, 1.0âmm lateral from bregma). Guide cannula was positioned 1.3âmm above the injection site (1âmm ventral to the surface of the skull). AgRP-Cas9 mice were treated with either AAV-gScrambled or AAV-gIR and underwent guide cannula placement 7 days post AAV administration. Mice were administered intracerebroventricular vehicle (ddH2O), fluorosamine (100âµg per day or 250âµg per day) in a volume of 2âµl and all compounds were delivered approximately 1âh before lights off (19:00).
Intranasal drug delivery
Conscious mice were restrained by scruffing and inverted parallel to the floor with the chin at ~180-degree angle with the neck. Using a 10âµl tip, a pipettor was loaded with 5âµl of vehicle (ddH2O) or fluorosamine (1âmg per mouse in 20âµl or 5âmg per mouse in 20âµl). The tip of the filled pipettor was placed near the left nostril at a 45-degree angle, and the drug was ejected to form a small 5âµl droplet at tip for the mouse to inhale. Immediately after the mouse inhaled the first droplet the remaining solution was ejected to form another small droplet for the mouse to inhale through the same nostril. The mouse was held in this position for 15âs before the procedure was repeated in the right nostril. The mouse was returned to the cage for 2âmin and the process was repeated so that each mouse received four droplets of 5âµl each, delivering a total of 20âµl of solution. All drugs were administered delivered approximately 1âh before lights off (19:00).
PNN tracker validation and quantification
To determine PNN turnover in the ARC, RSG, or CC, mice received stereotaxic injections of WFAâbiotin as described in âStereotaxic surgeryâ. At experimental endpoints mice were transcardially perfused and assessment of pulse labelled ARC PNN was identified by immunofluorescent detection of WFAâbiotin (PNN at the time of pulse) and WFAâFITC (total PNN) as described in âImmunohistochemistryâ.
To chase the pulsed WFAâbiotin in the ARC, sections were imaged and analysed using ImageJ v1.53âs (NIH) software. Raw images underwent background subtraction using a rolling ball algorithm to minimize background and tissue autofluorescence. To quantify staining area within the ARC, images were thresholded and binarized to create ROI masks for WFAâbiotin and WFAâFITC. For each image, staining ROI area (µm2) and intensity was calculated.
To validate the PNN tracker technique, C57BL/6J mice were stereotaxically injected unilaterally with WFA (0.3âµg per side, in a volume of 150ânl) to pulse the PNN into one side of the ARC and saline injected into the other side. One day later mice were transcardially perfused and ARC brain sections were stained and analysed for PNN tracker analysis. To determine how faithfully the pulsed WFAâbiotin represents the current PNN we quantified the percentage area to which WFAâbiotin (pulse labelled) co-localizes with WFAâFITC (total present PNN).
To validate that the chased WFAâbiotin signal represents bona fide PNN staining we stereotaxically injected WFA (0.3âµg per side, in a volume of 150ânl) bilaterally into the ARC of 8-week-old C57BL/6J mice. 3 days later mice received unilateral ARC injections of chABC (15 mU per side in a volume of 150ânl) or vehicle to disassemble the WFAâbiotin bound PNN. To determine the specificity of pulsed WFAâbiotin we quantified and compared the area and intensity of WFAâbiotin staining in the chABC and vehicle-treated sides of the ARC.
To determine PNN turnover in lean and obese mice, we stereotaxically injected WFAâbiotin (0.3âµg per side, in a volume of 150ânl) bilaterally into the ARC of 12-week HFHS-fed C57BL/6J mice or aged-matched controls. Brains were extracted either the day after surgery (day 0) or following 1, 3, 5 and 10 weeks post-injection. Brain sections were stained for the presence of WFAâbiotin and WFAâFITC, and we quantified the area of WFAâbiotin staining as described above. To determine PNN turnover we compared WFA-labelled PNN present at the start of the experiment (day 0) to that which remained at weeks 1, 3, 5 and 10. WFAâFITC labelling of the PNN was performed at each time point to validate the presence of the ARC PNN and ensure changes in WFAâbiotin labelling were not due to loss of the PNN over time. The same process was used to assess turnover in the RSG and blood vessels of the CC.
ARC CS-GAG and hyaluronic acid disaccharide quantification
Microdissected ARC tissues were incubated in the extraction buffer, containing 8âM urea, 0.5% Triton X-100, 5âmM Tris 2-carboxyethylphosphine and cOmplete mini ETDA-free protease inhibitor cocktail (Merck) for 30âmin with gentle mixing and then homogenized. Samples were centrifuged for 30âmin at 5,000ârpm and the supernatant was collected and buffer exchanged using Amicon Ultracell-10k MWCO centrifugal tubes into PBS. Protein concentration of each sample was estimated using Bradford assay. Thirty μg of each protein extract was reduced using 5âmM dithiothreitol for 30âmin at 50â°C and alkylated with 10âmM iodoacetamide for an hour at room temperature before blotting onto 0.45 μm PVDF membrane (Millipore, IPVH20200) and dried. Each sample spot was transferred into a 96-well plate and blocked using 1% (v/v) polyvinylpyrrolidone solution in water.
The disaccharide analysis procedure was adapted from53 with the following modifications. GAG disaccharides were released from the PVDF sample spots using an enzyme mix containing 5 mU chABC (Sigma, Cat# C3667), 50âng each of heparinase I/II/III (R&D Systems) in 100âmM ammonium acetate pH 7 with 5âmM calcium chloride and incubated at 30â°C overnight. An additional mixture of purified GAG polysaccharides containing 1âμg each of bovine kidney heparan sulfate (Sigma-Aldrich, H7640), 10âμg shark chondroitin sulfate (Signma-Aldrich, C4382) and 1âμg of Streptococcus equi hyaluronic acid (Sigma-Aldrich, 53747) were digested alongside samples as enzyme reaction controls, and as retention time standards. Digested disaccharides were collected and dried under low pressure for labelling using 2-AB (2-aminobenzamide), according to a commercially available protocol (Ludger LT-KAB-VP24-Guide-v2.0). Samples, alongside a standard mix of 8 common HS (Iduron, HS mix) and 8 common chondroitin sulfate disaccharides (Iduron, chondroitin sulfate mix), were labelled with 2-AB and washed with octanal twice to remove excess labelling agent. Cleaned samples in the aqueous layer were dried and resuspended in 75% (v/v) acetonitrile with 10âmM ammonium acetate, pH 6.8.
The labelled disaccharides were separated by liquid chromatography using a SeQuant ZIC-HILIC column (200âà pore size, 3.5âµm particle size, 1âmmâÃâ150âmm) at 35â°C using an Agilent 1260 Infinity II with fluorescence detection. The mobile phase solvent A (10âmM NH4Ac, pH 6.8) and solvent B (90% acetonitrile in 10âmM NH4Ac pH 6.8) were run at a constant flow rate of 60âμlâminâ1 in microflow mode with gradient parameters as follows: 0â3âmin, 100% B; 4â14âmin, 94% B; 34âmin, 86% B; 47 min, 75% B; 51âmin, 60% B; 52â57 min, 60% B; 58ââ65 min, 100% B. Fluorescence detection was carried out with excitation and emission wavelengths set at 320ânm and 420ânm, respectively. Peaks were identified using the standard panel and polysaccharide digest control as retention time standards and the abundances were quantified manually by peak area.
Patch clamp electrophysiology
Npy-GFP male mice were placed on a HFHS diet for 12 weeks before being stereotaxically injected with either vehicle or chABC in the ARC 3 days before electrophysiological characterization. Mice were anaesthetized with isoflurane prior to brain extraction, and brains were incubated in ice-cold artificial cerebrospinal fluid (aCSF) of the following composition: 127âmM NaCl, 1.2âmM KH2PO4, 1.9âmM KCl, 26âmM NaHCO3, 3âmM D-glucose, 7âmM mannitol, 2.4âmM CaCl2, 1.3âmM MgCl2 (saturated with 95% O2 and 5% CO2, pH 7.4). Coronal sections (250 μm) of the ARC were cut using a vibratome (Leica VTS1000S). Slices were heated for 30âmin at 34â°C and then allowed to cool to room temperature prior to recording. Slices were placed in a recording chamber and continuously perfused with room temperature aCSF.
Npy-GFP neurons in the ARC were visualized using fluorescence and differential interference contrast optics with infrared video microscopy (AxioCam MRm, Zeiss) and an upright microscope (BX51WI, Olympus). For current clamp recordings, patch pipettes (8â11 MΩ) were pulled from thin-walled borosilicate glass (Sutter Instruments, BF150-86-10) using a horizontal puller (Sutter Instruments) and filled with intracellular solution containing 140 mM potassium gluconate, 10âmM HEPES, 10âmM KCl, 1âmM EGTA, 4âmM Na-ATP, 0.3âmM Na-GTP and 10âmM Biocytin (300âmOsm and pH 7.3, with osmolality and pH adjusted with sucrose and KOH accordingly). In voltage-clamp recordings to examine K+ currents, patch pipettes (3â6 MΩ) were filled with intracellular solution containing 130 mM potassium gluconate, 6âmM NaCl, 4âmM NaOH, 11âmM EGTA, 1âmM CaCl2, 10âmM HEPES, 1âmM MgCl2, 2âmM Na-ATP, 0.2âmM Na-GTP, 0.1% biocytin (295âmOsm and pH 7.3, with osmolality and pH adjusted with sucrose and KOH accordingly). Cells with a series resistance of >20 MΩ were not included in the analysis. Recordings were made in the presence of tetrodotoxin, where 11 depolarizing pulses applied from â40 to +60âmV for 500âms in 10âmV increments from a holding potential of â80âmV. A 50âms prepulse to 0âmV was used to inactivate any residual voltage-dependent Na+ current. Whole-cell recordings were made using a Double IPA Integrated Patch amplifier controlled with SutterPatch software (Sutter Instruments) with all current clamp data filtered at 5âkHz. Data were analysed using Sutterpatch (Sutter Instruments) and Clampfit 10.7 (Axon Instruments).
Immunoblotting
The mediobasal hypothalamus was microdissected and snap frozen in liquid nitrogen. Tissues were mechanically homogenized in 100âμl ice-cold RIPA lysis buffer (ab156034, Abcam, UK, containing PhosStop Phosphatase Inhibitor, 1 tablet per 10âml; Roche PHOSS-RO) and clarified by centrifugation (13,000ârpm for 20âmin at 4â°C). Tissue lysates were resolved by SDSâPAGE and immunoblotted as described previously (PMID: 31509751). Antibodies used are rabbit phospho-IR (Tyr1162, Tyr1163) polyclonal antibody (1:1,000, 44â804âG, Invitrogen, MA), rabbit monoclonal anti-IR (1:1,000, 3025x, Cell Signaling), rabbit-β-actin polyclonal antibody (1:2,000, 4967, Cell Signaling Technology), mouse GAPDH monoclonal antibody (1:5,000, 60004-1-Ig, Proteintech), mouse monoclonal anti-tubulin (1:2,000, T5168, Sigma).
Real-time PCR
RNA was extracted using TRIzol reagent (Invitrogen) and total RNA quality and quantity determined using a NanoDrop 3300 v2.8.1 (Thermo Scientific). mRNA was reverse-transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and processed for quantitative real-time PCR using SYBR Green PCR Master Mix (4309155, Applied Biosystems). The following primers were used for SYBR green expression assays: Adamst4 (forward-GAACGGTGGCAAGTATTGTGAGG, reverse-TTCGGTGGTTGTAGGCAGCACA), Adamst5 (forward-CTGCCTTCAAGGCAAATGTGTGG, reverse-CAATGGCGGTAGGCAAACTGCA), Ikkb (forward-GCAGACTGACATTGTGGACCTG, reverse-ATCTCCTGGCTGTCACCTTCTG), Il6 (forward-GGTGCCCTGCCAGTATTCTC, reverse-GGCTCCCAACACAGGATGA), Kcna4 (forward-GCAGATTGCTGAATGACACCTCG, reverse-GGACAAGCAAAGCATCGAACCAC), Kcnb1 (forward-GAGGAGTTCGACAACACGTGCT, reverse-TGAGTGACAGGGCAATGGTGGA), Kcnb2 (forward-GCTGGAGAAACCTAACTCGTCC, reverse-CTCGTCGTTTTCTTGCAGCTCTG), Kcnc3 (forward-GAAGAGGTGATTGAAACCAACAGG, reverse-TGGGCTCTTGTCTTCTGGAGAC), Kcnc4 (forward-CCAGCTCGAATCGCCCATTTAC, reverse-AGCACCGCATTAGCATCGCCAT), Kcnd2 (forward-CCTACATGCAGAGCAAGCGGAA, reverse-GTGGTTTTCTCCAGGCAGTGAAG), Kcnd3 (forward-AGAAGAGGAGCAGATGGGCAAG, reverse-CTTGATGGTGGAGGTTCGTACAG), Kcnj11 (forward-TGCGTCACAAGCATCCACTCCT, reverse-GGACATTCCTCTGTCACCATGC), Kcnj3 (forward-CAGTTCGAGGTTGTCGTCATCC, reverse-CCCAAAGCACTTCGTCCTCTGT), Kcnj6 (forward-GGAACTGGAGATTGTGGTCATCC, reverse-TCTTCCAGCGTTAGGACAGGTG), Kcnj9 (forward-TCTCACCTCTCGTCATCAGCCA, reverse-GCTTCGAGCTTGGCACGTCATT), Kcnma1 (forward-CCTGAAGGACTTTCTGCACAAGG, reverse-ACTCCACCTGAGTGAAATGCCG), Kcnn3 (forward-TCCACCGTCATCCTGCTTGGTT, reverse-CAGGCTGATGTAGAGGATACGC), Kcnq3 (forward-AAGCCTACGCTTTCTGGCAGAG, reverse-ACAGCTCGGATGGCAGCCTTTA), Mmp13 (forward-AGCAGTTCCAAAGGCTACAACT, reverse-GGATGCTTAGGGTTGGGGTC), Mmp14 (forward-AGCACTGGGTGTTTGACGAA, reverse-CCGGTAGTACTTATTGCCCCG), Mmp2 (forward-GTCGCCCCTAAAACAGACAA, reverse-GGTCTCGATGGTGTTCTGGT), Mmp9 (forward-GCTGACTACGATAAGGACGGCA, reverse-TAGTGGTGCAGGCAGAGTAGGA), Nfkb1 (forward-GCTGCCAAAGAAGGACACGACA, reverse-GGCAGGCTATTGCTCATCACAG), Rn18s (forward-CAGCTCCAAGCGTTCCTGG, reverse-GGCCTTCAATTACAGTCGTCTTC), sTgfβr2 (forward-AAGGGTTCAGCCTACACCTT, reverse-GTCGGGACTGCTGGTGGTGT), sTnfr1α (forward-GGTTATCTTGCTAGGTCTTTG, reverse-GATCCCTACAAATGATGGAG), Tgfb1 (forward-GGATACCAACTATTGCTTCAG, reverse-TGTCCAGGCTCCAAATATAG), Tgfb2 (forward-CTAATGTTGTTGCCCTCCTACAG, reverse-GCACAGAAGTTAGCATTGTACCC), Tgfbr1 (forward-GGACCATTGTGTTACAAGAAAGC, reverse-CATGGCGTAACATTACAGTCTGA), Tgfbr2 (forward-TCCTAGTGAAGAACGACTTGACC, reverse-TACCAGAGCCATGGAGTAGACAT), Timp1 (forward-TCTTGGTTCCCTGGCGTACTCT, reverse-GTGAGTGTCACTCTCCAGTTTGC), Timp3 (forward-GCTAGAAGTCAACAAATACCAG, reverse-TAGTAGCAGGACTTGATCTTG) and Tnf (forward-CTGTGAAGGGAATGGGTGTT, reverse-GGTCACTGTCCCAGCATCTT).
Gene expression was normalized to Rn18s and relative quantification was achieved using the ÎÎCT method. Reactions were performed using a Bio-Rad CFX 384 touch (Bio-Rad).
PNN binding assay
To determine the interaction of insulin with PNN components in vitro, flat-bottom 96-well plates were first coated with 10âμgâmlâ1 poly-l-lysine overnight, followed by a rinsing with water. A purified CSPGs mix containing neurocan, phosphacan, versican and aggrecan (CC117, Merk Millipore), purified aggrecan (A1960, Merk Millipore) or purified C4S (S9004, Selleck Chemicals), were coated onto the 96-well plates at a concentration of 10âμgâmlâ1 for 4âh at room temperature, followed by a rinse with water. InsulinâFITC was incubated on plates containing ECM at concentration ranging from 5â1âmgâmlâ1 for 2âh at room temperature and protected from light. Control wells contained either no ECM, bovine serum albumin (10âμgâmlâ1) or poly-l-lysine alone. Wells were washed 3 times with water and imaged using a SPECTROstar Nano Microplate Reader (BMG Labtech, Germany). To digest PNN or to negate PNN negative charge, wells were incubated with either chABC (0.5âUâmlâ1) or poly-l-arginine (10âμgâmlâ1, P7762, Merk Millipore) for 1âh at 37°âC after the ECM coating, washed 3 times with water and then incubated with insulinâFITC.
Statistics and Reproducibility
Statistical analyses were performed using GraphPad Prism version 10 (GraphPad Software). Statistical significance was determined by a one-way or two-way ANOVA with multiple comparisons or repeated-measures, one or two-tailed paired or unpaired Studentâs t-tests, ANCOVA, or simple linear regression as appropriate. P <â0.05 was considered significant: *Pâ<â0.05, **Pâ<â0.01 and ***Pâ<â0.001. Statistical details of individual experiments such as exact values of n and exact statistical tests can be found in figures and legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.