Animals
All experimental methods and animal husbandry for transgenic quails were performed in accordance with the guidelines of the European Union 2010/63/UE, approved by the Institut Pasteur ethics committee authorization #dha210003, and under the GMO agreement 2432.
Embryo imaging, orientation and laser dissection
Transgenic memGFP quail embryos6 were collected at stage XI using a paper filter ring and cultured on a semi-solid nutritive medium of thin chicken albumen, agarose (0.2%), glucose and NaCl, as described previously6. The embryos were then transferred to a glass-bottom six-well plate (Mattek) with 2âml of the nutritive medium and imaged at 38â°C using the Zeiss LSM 900 microscope with Ã2.5 or Ã5 objectives. The time interval between two consecutive frames was 6âmin. Sample size was not predetermined. The selection of embryos used in experiments was not randomized and blinding was not used for the analyses.
For bisection experiments, epiblasts were oriented by analysing tissue flows in real-time using PIV. After 1â2âh of image acquisition, the margin, the embryonic and extraembryonic territories, and the presumptive anteriorâposterior axis of the embryo were determined automatically, as previously described6. Next, the spatial xây coordinates of a line passing through the centre of the epiblast, with a specific angle to the anteriorâposterior axis (as explained in Extended Data Fig. 4), were obtained and transferred to the ROE SysCon software of the laser dissector. Laser severing was performed using the UGA-42 firefly module coupled to a 355ânm pulsed laser (100% power) from Rapp Optoelectronic and the above-mentioned microscope and objectives. After bisection, the two halves of the embryo were moved on two separate vitelline membranes, or one half was left in its position and the other half was gently removed with a mouth pipette.
To ensure accurate comparison between posterior halves with fixed/free borders and intact epiblasts, their developmental stage was precisely matched. As described previously6, embryos were staged according to the integrated contraction of a posterior segment of the margin. This was evaluated on the fly based on PIV analysis of tissue motion. Embryos exhibiting an identical contraction (20%, reached in around 2âh) were bisected and allowed to develop until they contracted by the same amount (50%, reached in about 3âh). The equivalent stage for control (intact) embryos was determined by calculating the total contraction (before + after the cut) of posterior halves (60%; around 5âh). Thus, embryos presenting the same amount of tissue converging towards the primitive streak are being compared, ruling out possible confounding effects due to advection. For simplicity, hours only were reported in figures.
Attachment of the embryo was favoured by cooling down the embryo at room temperature for 1âh and removing excess liquid culture medium between the freshly cut edge and the vitelline membrane, whereas attachment was disfavoured by imaging posterior halves immediately after the bisection. Embryos were subjected to a second laser dissection if the edges reattached, as in Extended Data Fig. 5 and Supplementary Video 6.
Pharmacological treatment and obstacle
For the drug delivery experiments, 0.8% DMSO (0.8% v/v), calyculin A (Tocris, 35ânM), H1152 dihydrochloride (Tocris, 25-75âµM) and Ski-1 (Tocris, 75-125âµM) were added to the culture medium. For the obstacle experiment, memGFP quail embryos were oriented by eye, and a fragment of hair, or a Nylon wire coated with Cell-Tak (diameter, ~100âµm; length, ~6,000âµm) was gently deposited onto the ventral side of the embryo.
In situ hybridization and immunostaining
Quail embryos were fixed in ice-cold 4% formaldehyde, dehydrated in PBST (PBS/0.1% Tween-20) with increasing methanol concentrations (25%, 50%, 75% and 100%), and then rehydrated. Hybridization with DIG-labelled RNA probes was performed overnight at 65â°C in hybridization buffer (5à SSC pHâ4.5, 50% formamide, 1% SDS, 50âmgâmlâ1 yeast tRNA, 50âmgâmlâ1 heparin). The next day, the embryos were thoroughly washed and treated for 6âh with a blocking solution of MABT, 2% BBR (Boehringer blocking reagent) and 20% lamb serum. The embryos were then incubated overnight with an AP-coupled anti-DIG antibody (Biotechne, MAB7520, 1:2,000) in the blocking solution and finally stained with NBT/BCIP liquid substrate (Sigma-Aldrich). After the staining, the embryos were temporarily mounted onto a slide and photographed at different magnification using the SteREO Discovery.V8 system (Zeiss; equipped with an AxioCam MRc (Zeiss)). For the DIG-labelled probe, an 807âbp fragment of the SNAI2 coding sequence (from nucleotides 165 to 971) was PCR-amplified from quail cDNA and cloned into the pGEM-T Easy vector (Promega). The embryos were stained with antibodies against BRA (Biotechne, AF2085, 1:200).
RNAscope
The samples were fixed overnight at 4â°C in ice-cold 4% formaldehyde and then washed in PBST for 6âh. Staining was performed using the RNAscope Multiplex Fluorescent Reagent Kit V2 according to the manufacturerâs instructions with the following modification for whole-mount staining of quail blastoderm embryos. For sample pretreatment, no retrieval or proteinase treatment was performed. The probes used for the stainings were as follows: RNAscope Probe Cja-GDF1 (Bio-Techne, 593421), RNAscope Probe Cja-T-C2 (Bio-Techne, 587391-C2) and RNAscope Probe Cja-LOC107312850-C3 (Bio-Techne, 587381-C3), and were detected with the Opal520, Opal570 and Opal650 reagents (Perkin Elmer, 1:750 in TSA Buffer). The embryos were then mounted between the slide and coverslip using Fluoromount-G mounting medium (00-4958-02).
Quantification of gene expression and contraction along the margin
To relate tissue deformation and gene expression, images of fixed samples were aligned with the last timepoint from live imaging. The RNA levels along the embryo margin (Fig. 4t,u and Extended Data Fig. 5g) were measured by integrating the maximum-z-projected signal across a 400-µm-wide strip centred on the margin. The levels were normalized for each embryo to ~1 and ~0 inside and outside, respectively, the expression domain. The instantaneous size of the contracting domain of the margin (Fig. 4s) was determined from the profile of tangential velocity along the margin (see Fig. 4f,n), as the interval between the extrema of the velocity. The integrated contraction of a margin portion (as in Fig. 4u and Extended Data Fig. 5g) was defined as the logarithm of its fold change in length over the course of the experiment, normalized to the logarithm of the target contraction for that experiment (as specified above, 50% reduction in length for epiblast halves and 60% reduction for intact epiblasts; the sign is chosen such that the normalized contraction is around â1 in the posterior). The relative sizes of the GDF1 domain and contracted domain (Fig. 4v) were defined by thresholding the spatial profiles of GDF1 and contraction (see Fig. 4t and Extended Data Fig. 5g); the thresholds were taken to correspond to the midpoint of the approximately linear relationship between contraction and GDF1 in Fig. 4u (normalized expression >1/2, normalized contraction <â1/4).
Model
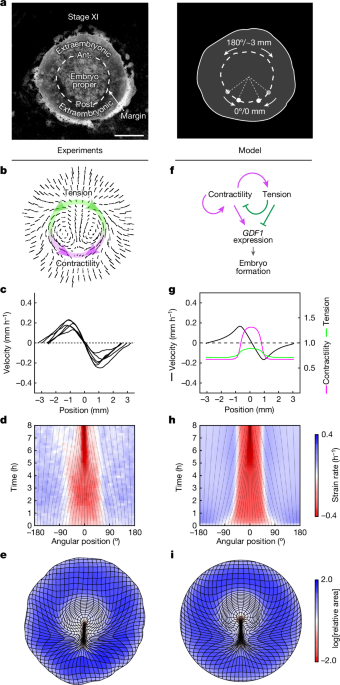
We considered a hierarchy of models to explore self-organization of force generation along the embryo margin through feedback of tissue tension on contractility. In the simplest instance, the margin is described as a 1D tensile line with a fixed length and periodic boundary conditions, and the surrounding tissue is ignored, corresponding to a limit at which force transmission along the margin dominates over force transmission to the surrounding tissue. We can further take a limit at which advection along the margin is negligible, which is the relevant regime for the embryo (regulation occurs on timescales that are shorter than the characteristic timescale for advection, which is the timescale over which the primitive streak emerges).
In the context of this minimal model, we identify contractility with the active tension Ta generated at the margin. Assuming that this active tension combines with a linear viscous resistance to stretching, the total tension T(s) at a point s along the margin is given by
$$T(s,t)={T}_{{\rm{a}}}({\rm{s}},{\rm{t}})+\nu \dot{{\varepsilon }}({\rm{s}},{\rm{t}})$$
where \(\dot{\varepsilon }\) denotes the strain rate (the local elongation rate of the margin) and ν is a 1D viscosity. Assuming that the active tension varies in response to the strain rate, we write
$$\frac{{\rm{\partial }}{T}_{{\rm{a}}}}{{\rm{\partial }}t}+u\frac{{\rm{\partial }}{T}_{{\rm{a}}}}{{\rm{\partial }}s}=\frac{1}{\tau }\left[{T}_{0}\left(1+\zeta \,{\rm{t}}{\rm{a}}{\rm{n}}{\rm{h}}\left(\alpha -\beta \frac{v}{{T}_{0}}\dot{{\varepsilon }}\right)\right)-{T}_{{\rm{a}}}\right]+D\frac{{{\rm{\partial }}}^{2}{T}_{{\rm{a}}}}{{\rm{\partial }}{s}^{2}}$$
where Ï is a characteristic timescale for regulation, and we have included a diffusion term with diffusivity D to account for non-local self-activation of contractility, which might arise from the recruitment of neighbouring cellâcell junctions into cables that span several cells and are constantly turning over within the margin.
With the surrounding tissue being neglected, mechanical balance implies that the tension T is uniform along the margin, and conservation of the total margin length implies that the average strain rate vanishes, \(\left\langle \dot{\varepsilon }\right\rangle =0\), so that
$$\frac{{\rm{\partial }}{T}_{{\rm{a}}}}{{\rm{\partial }}t}+u\frac{{\rm{\partial }}{T}_{{\rm{a}}}}{{\rm{\partial }}s}=\frac{1}{\tau }\left[{T}_{0}\left(1+\zeta \tanh \left(\alpha +\beta \frac{{T}_{{\rm{a}}}-\langle {{\rm{T}}}_{{\rm{a}}}\rangle }{{T}_{0}}\right)\right)-{T}_{{\rm{a}}}\right]+D\frac{{{\rm{\partial }}}^{2}{T}_{{\rm{a}}}}{{\rm{\partial }}{s}^{2}}$$
Thus, active tension is upregulated and downregulated where it is above and below, respectively, its spatial average. Mathematically, this model is equivalent to a molecular activatorâinhibitor model, in which the tension Tâ=ââ¨Taâ© serves as a long-range inhibitor, in the limit of infinitely fast and long-range inhibitor diffusion. If the coefficient β representing the strength of mechanical feedback is large enough, the model supports spontaneous symmetry breaking and the stable maintenance of regions of high and low contractility. In the relevant parameter regime, the steady state of the model is governed by the motion of narrow fronts between these regions that tend towards invariant proportions, corresponding to a fixed total tension. When advection is taken into account, the fronts are displaced to a position where advection is balanced by the tendency to return to this preferred, homeostatic tension. Details of the model and its analysis are provided in the Supplementary Discussion.
Our full 2D model incorporates the same mechanical regulation of contractility into our previously described fluid-mechanical model of tissue flows in the embryo6. In brief, the embryonic disk is described as a 2D viscous fluid driven by tension along the margin, with a prescribed divergence term γ that allows for non-uniform areal expansion of the embryonic disk. The profile of stresses imparted on the tissue by the margin is assumed to keep an invariant, Gaussian profile, such that distributed force generation at the margin and its regulation can still be described in terms of a 1D profile of contractility. To allow for a nonlinear relationship between mechanical load on cellâcell junctions and contraction rate, and a saturation of the contraction rate, the margin is explicitly described as a 1D viscoelastic line; the rest length l0 of an element of margin varies as
$$\frac{1}{{l}_{0}}\frac{{\rm{d}}{l}_{0}}{{\rm{d}}t}=\lambda {\dot{{\varepsilon }}}_{0}W\left(\frac{T}{c{T}_{{\rm{s}}}};\lambda \right)+\frac{\gamma (x,t)}{2}$$
where c denotes the contractility (which is no longer identified with an active tension but could be understood to represent the local density of active myosin or supracellular cables), W is a nonlinear saturating âwalking kernelâ (see ref. 24) and the term γ/2 is included to allow for the shrinking of cables through cell ingression (corresponding to γâ<â0) at the primitive streak (see ref. 6). Ts denotes a stall force per unit contractility at which junctions transition from contraction to yielding, and the parameter λ controls the nonlinearity of the walking kernel. With E denoting the elastic modulus of the margin, we obtain the system of equations
$$\begin{array}{c}\frac{{\rm{\partial }}c}{{\rm{\partial }}t}+u\frac{{\rm{\partial }}c}{{\rm{\partial }}s}=\frac{1}{\tau }\left[{c}_{0}+\Delta c\,\tanh \left(\alpha -\frac{\beta \dot{{\varepsilon }}}{\lambda {\dot{{\varepsilon }}}_{0}}\right)-c\right]+D\frac{{{\rm{\partial }}}^{2}c}{{\rm{\partial }}{s}^{2}}\\ \frac{{\rm{\partial }}T}{{\rm{\partial }}t}+u\frac{{\rm{\partial }}T}{{\rm{\partial }}s}=E\left[\dot{{\varepsilon }}-\frac{\gamma }{2}-\lambda {\dot{{\varepsilon }}}_{0}W\left(\frac{T}{c{T}_{{\rm{s}}}};\lambda \right)\right]\end{array}$$
where T is the elastic tension along the margin, which determines the velocity u through the Stokes equation describing tissue motion.
The prescribed area changes, which approximate experimentally observed area changes using analytic functions according to a previous study6, include contributions from expansion of extraembryonic tissue and ingression at the primitive streak. Here, the contribution of ingression at the primitive streak is included when modelling the intact epiblast (Fig. 1), to most closely compare with the model from ref. 6 in which both area changes and active forces were prescribed, as well as for the asymmetric perturbations in Extended Data Fig. 8, which have a limited effect on primitive streak formation. On the other hand, this contribution is omitted in simulations of posterior halves (Fig. 4) and with a full obstacle (Fig. 5), as we do not wish to explicitly model the redirection of cell ingression when primitive streak formation is displaced; this is inessential for our purposes, as our focus in on the redirection of force generation and tissue flows upstream of primitive streak formation, and as ingression makes a limited contribution to shaping the embryo in the time interval considered here, as quantified previously6.
This 2D model is used to simulate the full course of margin regulation and tissue flows within the embryonic disk upon perturbations, and in a simplified geometry is amenable to a similar analytical description as the minimal 1D model. For numerical simulations, it was implemented in Python, using the FEniCS finite element platform33,34. A detailed discussion is provided in the Supplementary Methods.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.