Animals
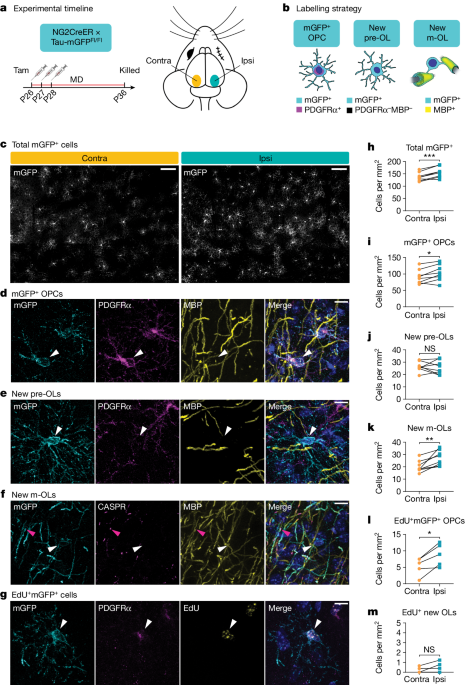
All mice were handled in accordance with, and all procedures approved by, the Institutional Animal Care and Use Committee of the University of California San Francisco. Mice were group housed (between two and five per cage) throughout all experiments and given food and water ad libitum on a 12/12âh light/dark cycle in a temperature-controlled (22â24â°C) and humidity-controlled (40â60%) environment. Housing conditions adhered to the standards maintained by University of California San Francisco Institutional Animal Care and Use Committee, which include standard-sized mouse cages, bedding, nestlet, gnawing block and Enviro-dry nesting material. No additional environmental enrichment was provided. Males and females were used for all experiments. For tracking of oligodendrogenesis during adolescence, NG2CreER:tau-mGFP mice51,52 (Jax, nos. 008538 and 021162) received 100âmgâkgâ1 tamoxifen (Sigma, catalogue no. T5648) by oral gavage from P26 to P28. A subset also received 80âmgâkgâ1 EdU (Carbosynth, catalogue no. NE08701) by intraperitoneal injection at the same time. For blocking of adolescent oligodendrogenesis, Pdgfra-creER:MyrfFl/Fl mice13,53 (Jax, nos. 018280 and 010607) received 100âmgâkgâ1 tamoxifen by oral gavage from P10 to P14. For visualization of dendritic spines in vivo, Pdgfra-creER:MyrfFl/Fl mice were crossed with Thy1-YFP-H mice54 (Jax, no. 003782). Experimenters were blinded to animal genotype throughout data acquisition and analysis.
Immunohistochemistry
Mice were deeply anaesthetized with Avertin and perfused transcardially with 4% paraformaldehyde in 1ÃâPBS. Brain tissue was isolated and postfixed in this solution overnight at 4â°C, then stored in 1ÃâPBS with 0.1% NaAz. Brains were sucrose protected (30% in PBS) before flash-freezing and sectioning coronally (30âμm) on a sliding microtome. Free-floating sections were permeabilized/blocked with 0.2% Triton X-100 and 10% normal goat serum in 1ÃâPBS for 1âh at room temperature. Sections were incubated with primary antibodies prepared in 0.2% Triton X-100 and 10% normal goat serum in 1ÃâPBS at 4â°C overnight. Sections were incubated with secondary antibodies in 10% normal goat serum in 1ÃâPBS for 2âh at room temperature. Primary antibodies and concentrations used are as follows: rabbit anti-ASPA (1:1,000), chicken anti-GFP (1:1,000), rat anti-MBP (1:200), rabbit anti-PDGFRα (1:200), rabbit anti-cleaved caspase-3 (1:200), mouse anti-glial fibrillary acidic protein (1:1,000), human anti-SOX9 (1:2,000), rabbit anti-IBA1 (1:1,000), mouse anti-NF-L Degenotag (1:1,000), rabbit anti-NF-H (1:1,000), mouse anti-PV (1:1,000), biotinylated WFA (1:400), rabbit anti-CASPR (1:600) and mouse anti-BCAS1 (1:300); additional details are listed in Supplementary Table 2. The primary antibodies above have been validated for use in immunohistochemistry in mouse tissue, in published literature and on the manufacturerâs websites. Secondary antibodies used included the following: Alexa Fluorâ488-, 594- or 647-conjugated secondary antibodies to rabbit, mouse, human, chicken, rat or streptavidin (1:1,000, all raised in goat; purchased from Thermo Fisher Scientific or Jackson ImmunoResearch); additional details are listed in Supplementary Table 2. Cell nuclei were labelled with DAPI (Vector Laboratories). TUNEL immunostaining was performed on fixed brain sections according to the manufacturerâs instructions using the Abcam TUNEL Assay KitâBrdU-Red (abcam, catalogue no. ab66110).
Fixed-tissue imaging and analysis
Tiled z-stacks (with 2âµm steps) spanning either 30âµm sections of visual cortex and lateral geniculate nucleus or 20âµm sections of optic nerve were taken with a Zeiss Axio Imager Z1 with ApoTome attachment and Zeiss Zenâ2 (blue edition, v.2.0.0.0) software, using a Ã10âobjective. For quantification, images were taken from two or three sections per mouse. Cell density was quantified manually using Cell Counter in Fiji. Experimenters were blinded to genotype throughout imaging acquisition and analysis.
Slice electrophysiology
Mice aged 8â12âweeks were anaesthetized with isofluorane. Brains were quickly removed and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing 125âmM NaCl, 2.5âmM KCl, 2âmM CaCl2, 1.25âmM NaH2PO4, 1âmM MgCl2, 25âmM NaHCO3 and 15âmM d-glucose. ACSF was saturated with 95% O2 and 5% CO2. Osmolarity was adjusted to 300â305âmOsm. Coronal sections (300âµm) containing visual cortex were prepared in ice-cold ACSF using a vibrating-blade microtome (Leica VT1200). Slices were recovered for 20âmin at 32â°C and then transferred to ACSF at room temperature. Following the recovery period, slices were moved to a submerged recording chamber perfused with ACSF at a rate of 2â3âmlâminâ1 at 30â31â°C, and brain slices were recorded within 5âh of recovery. Voltage-clamp recordings of mIPSCs were made using glass pipettes of resistance 2â4âMΩ, filled with internal solution containing 126âmM CsMeSO3, 8âmM NaCl, 10âmM HEPES, 2.9âmM QX-314, 8âmM Na2-phosphocreatine, 0.3âmM GTP-Na, 4âmM ATP-Mg, 0.1âmM CaCl2 and 1âmM EGTA, pHâ7.2â7.3, osmolarity 285â290âmOsm. Input resistance was monitored online during recordings; cells with access resistance greater than 20âMΩ were excluded from analysis. Recordings were made at 0âmV holding potential. mIPSCs were pharmacologically isolated with tetrodotoxin (1âμM), NBQX (10âμM) and APV (50âμM) in the bath. Between 200 and 300âevents per cell were analysed using a threshold of 2Ãâbaseline noise. Recordings were obtained with a Multiclamp 700B amplifier (Molecular Devices) using WinWCP software (University of Strathclyde, UK). Signals were filtered at 2âkHz, digitized at 10âkHz (NI PCIe-6259, National Instruments) and analysed offline using the MiniAnalysis Program (Synaptosoft). The experimenter was blinded to the genotype of the animals throughout recording and analysis.
Monocular deprivation
Mice were anaesthetized using 5% isofluorane and anaesthesia was maintained with 2â3% isofluorane. The right eyelid was sutured closed by two mattress stitches, at either P26 for adolescent NG2CreER:tau-mGFP mice or 6â12âweeks for post-critical-period Pdgfra-creER:MyrfFl/Fl mice. Meloxicam and buprenorphine were administered before and after surgery for pain management. Animals were checked daily to ensure that the sutured eye remained closed for the required duration of the experiment. Sutures were removed just before postmonocular deprivation ISI sessions. Eyes were flushed with sterile saline and checked for clarity under a microscope. Only mice without corneal opacities or signs of infection were used.
ISI
Repeated optical imaging of intrinsic signals and quantification of ocular dominance were performed as previously described18. In brief, during recording, mice were anaesthetized with 0.7% isoflurane in oxygen applied via a home-made nose mask, supplemented with a single intramuscular injection of 20â25âµg chlorprothixene. Mice underwent a non-invasive procedure in which a headplate was fixed to the surface of the skull to enable head-fixed imaging, and images were recorded transcranially. Intrinsic signal images were obtained with a Dalsa 1M30 CCD camera (Dalsa) fitted with a 135âÃâ50âmm tandem lens (Nikon) and red interference filter (610â±â10ânm), using custom Linux software. Frames were acquired at a rate of 30 per second, temporally binned by four frames and stored as 512âÃâ512 pixel images following spatial binning of 1,024âÃâ1,024 camera pixels by 2âÃâ2 pixels. The visual stimulus for recording the binocular zone, presented on a 40âÃâ30âcm2 monitor placed 25âcm in front of the mouse, consisted of 2°-wide bars that were presented between â5 and 15° on the stimulus monitor (0°, centre of the monitor aligned to centre of the mouse) and moved continuously and periodically upward or downward at a speed of 10°âsâ1. The phase and amplitude of cortical responses at the stimulus frequency were extracted by Fourier analysis as previously described18. Response amplitude was taken as an average of at least four measurements. Ocular dominance index was computed as previously described18. In brief, the binocularly responsive region of interest (ROI) was chosen based on the ipsilateral eye response map following smoothing by low-pass filtering, using a uniform kernel of 5âÃâ5 pixels and thresholding at 40% of peak response amplitude. Ocular dominance score (CâââI)/(Câ+âI) was computed for each pixel in this ROI, in which C and I represent the magnitude of response to contralateral and ipsilateral eye stimulation, respectively, followed by calculation of ocular dominance index as the average of ocular dominance score for all responsive pixels. Experimenters were blinded to genotype throughout imaging and analysis.
CW surgery
At the age of 8â12âweeks, a square 3âÃâ3âmm2 cranial window (no.â1 coverslip glass, Warner Instruments) was placed over the left hemisphere of the cortex contralateral to the deprived eye. Mice were anaesthetized using 5% isofluorane and anaesthesia was maintained with 2â3% isofluorane. A craniotomy matching the size of the coverslip was cut using no.â11 scalpel blades (Fine Science Tools) and the coverslip carefully placed on top of the dura within the craniotomy without excessive compression of the brain. The window was centred using stereotactic coordinates 2âmm lateral and 3âmm posterior from bregma for visual cortex. The window and skull were sealed using dental cement (C&B Metabond, Parkell). A custom-made metal head bar was attached to the skull during surgery for head-fixed imaging. Mice were allowed to recover for 2â3âweeks before two-photon imaging.
In vivo longitudinal imaging
Longitudinal in vivo two-photon imaging was performed, as previously described31, with Pdgfra-creER:MyrfFl/Fl mice crossed with Thy1-YFP-H mice. Specifically, apical dendrites of cortical pyramidal neurons expressing YFP were imaged repeatedly 10â100âμm below the cortical surface through the cranial window in mice under isoflurane anaesthesia. Images were acquired using a BergamoâII two-photon microscope system with a resonant scanner (Thorlabs) and a Ã16/0.8 numerical aperture water-immersion objective lens (Nikon), using ThorImage LS software. YFP was excited at 925ânm with a mode-locked, tunable, ultrafast laser (InSightX3, Spectra-Physics) with 15â100âmW of power delivered to the back-aperture of the objective. Image stacks were acquired at 1,024âÃâ1,024 pixels with a voxel size of 0.12âμm in x and y and a z-step of 1âμm. Imaging frames from resonant scanning were averaged during acquisition to achieve a pixel dwell time equivalent of 1âns. Up to six imaging regions were acquired for each mouse. Representative images shown in the figures were created by making z-projections of three-dimensional stacks and were median filtered and contrast enhanced.
Analysis of in vivo spine imaging
Dendritic spines were analysed using the custom software Map Manager (https://mapmanager.net) written in Igor Pro (WaveMetrics) as previously described31,55. Experimenters were blinded to genotype throughout imaging acquisition and analysis. For annotation, the dendritic shaft was first traced using a modified version of the âSimple Neurite Tracerâ plug-in provided in ImageJ. Spine positions along a dendritic segment were manually identified by the location of the spine tip in three-dimensional image stacks of all imaging sessions. For longitudinal analysis, spines were further tracked across time by comparison of images from different sessions and connecting persistent spines. Rates of spine addition and elimination were calculated as the number of newly added or eliminated spines on a given imaging session divided by the total number of spines of that dendritic segment on the previous imaging session. The turnover ratio represents the sum of spine addition and elimination.
The fluorescence intensity of dendritic spines was used as a proxy for spine size, and therefore a three-dimensional ROI was defined for each spine, the dendritic shaft (4âμm stretch) adjacent to that spine and a nearby background region. For comparison of intensity values between imaging sessions, and to account for small variations in daily imaging conditions, spine signal intensity was normalized to the signal on the adjacent dendritic shaft following background subtraction. Each spine value was subsequently normalized to an average of the baseline imaging sessions by first subtracting the baseline value and then dividing over the sum of the baseline and respective imaging day value. This normalizes spine size change values between â1 and +1. All spine analysis was performed for each dendritic segment, averaged per genotype and is presented as the average of values from two adjacent imaging sessions (â3 and â2, â1 and 0, 1 and 2 and so on) to increase clarity.
For analysis of spine clustering, spines were classified as either increasing, decreasing or stable based on their average change in size on daysâ1â4 compared with baseline. The threshold for these categories was set based on the variability in control mice and was defined at baselineâ±â1âs.d. of size changes (±0.14). Nearest-neighbour analysis was calculated by finding the closest neighbour of every spine along each dendritic segment. Each nearest-neighbour pair was included once only in the dataset and pairs were excluded if their distance was either below 1.0âμm (to avoid overlapping ROIs) or above 3.5âμm. The fractions of nearest-neighbour spine pairs in which both spines increase, both decrease or changes occur in the same direction or in opposite directions were quantified to compare the degree of clustering between genotypes.
To test the statistical significance of clustering, nearest-neighbour analysis was performed on a pool of randomized spines in which spine size change values were randomly shuffled along all spine positions in each dendrite. A Monte Carlo Pâvalue was calculated by summing the tail of the histograms from 10,000âpools of randomized spine pairings in which the nearest-neighbour analysis resulted in spine pair fractions that exceeded the real observed value.
Statistics and reproducibility
All graphed values are shown as meanâ±âs.e.m. Statistical details of experiments (statistical tests used, statistical values, exact n values) are listed in Supplementary Table 1. The number of animals included in each experiment was based on standards established in the literature. Statistics were performed using GraphPad Prism. Statistical significance was defined as Pâ<â0.05. Tests for normality and equal variances were used to determine the appropriate statistical test to use. All reported t-tests were two-tailed, with Welchâs correction when group variances were significantly different. For experiments with more than two groups, one-way ANOVA was used; for experiments with more than one variable, two-way ANOVA was used; for experiments with repeated measurements from the same animals, two-way repeated-measures ANOVA was used. All representative images were selected from one of a minimum of three independently repeated experiments with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.