Plasmid constructs
Codon-optimized SARS-CoV-2 nsp3 and nsp4 sequences from the Wuhan-Hu-1 SARS-CoV-2 genome were amplified by the donor plasmids pEGFPN1-nsp3-EGFP and pmCherryN1-nsp4-mCherry, which were purchased from Addgene (plasmid numbers 165108 and 165132). The insertions nsp3 and nsp4 were assembled using overlap-extension PCR followed by insertion into a destination pcDNA3.1 vector containing an N-terminal TwinStrep-EGFP tag using the ClonExpress II One Step Cloning kit (Vazyme, C112) to produce pcDNA3.1-TwinStrep-EGFP-nsp3-nsp4. The presence of the EGFP tag allows the timely assessment of transfection efficiency and the subsequent DMV isolation procedure. Site-directed mutagenesis was introduced to the full-length construct by overlapping PCR. All of the constructs were confirmed by sequencing (BGI Genomics).
Cell culture and antibodies
Expi293F cells (Expi293 Expression System Kit, A14635) were purchased from Thermo Fisher Science and were cultured in OPM-293 CD05 medium (OPM Biosciences, 81075-001) supplemented with 100âUâmlâ1 penicillin and 100âUâmlâ1 streptomycin at 125âr.p.m., 37â°C and 5% CO2. Suspension cultures of HEK293F cells were grown to a density of about 3âÃâ106 cells per millilitre and transfected with plasmids using PEI MAX (Polysciences), which can achieve 60â70% transfection efficiency. At 18â20âh post-transfection, 2.5âmM sodium butyrate was added, and cell pellets were collected after about 72âh by centrifugation followed by washing with 1à phosphate-buffered saline (PBS). The cell pellets were frozen in liquid nitrogen and stored at â80â°C until further use. HEK293T cells and VeroE6 cells (Research Resource Identifier CVCL_0574) were cultured in Dulbeccoâs modified Eagle medium (Thermo Fisher Science) supplemented with 10% fetal bovine serum, 100âUâmlâ1 penicillin and 100âUâmlâ1 streptomycin at 37â°C and 5% CO2. HEK293T cells and VeroE6 cells were transfected with plasmids using Lipofectamine 3000 (Thermo Fisher Science, L3000015) according to the manufacturerâs instructions.
Co-immunoprecipitation and western blot analysis
HEK293T cells were transfected with the wild-type or mutant pcDNA3.1-TwinStrep-EGFP-nsp3-nsp4 plasmids. At 48âh post-transfection, HEK293T cells were washed with 1à PBS and lysed using ice-cold cell lysis buffer (50âmM Tris-HCl (pHâ7.5), 150âmM NaCl, 5% glycerol and 1% Triton X-100, supplemented with protease inhibitor cocktail (Sigma-Aldrich, P8340) and GENIUS Nuclease (ACROBiosystems, BEE-N3116)). After incubation on ice for 30âmin, cell debris was cleared by centrifugation at 12,000âr.p.m. for 30âmin at 4â°C. The supernatants were incubated with Strep-TactinXT 4Flow resin (IBA LifeSciences, 2-5010-025) for 3âh at 4â°C. The resin was then washed three times with ice-cold lysis buffer, and the bound proteins were eluted with 2à SDS sample buffer (0.2âM Tris-HCl (pHâ6.5), 0.4âM dithiothreitol, 8% SDS, 6âmM bromophenol blue and 4.3âmM glycerol). Samples were then subjected to SDSâpolyacrylamide gel electrophoresis (PAGE) and western blot analysis. Proteins were separated by SDSâPAGE and transferred to a polyvinylidene difluoride membrane (Millipore, IPVH00010). The membranes were subsequently blocked with 5% nonfat milk (Santa Cruz, sc-2325) in TBST (50âmM Tris-HCl (pHâ7.4), 150âmM NaCl and 0.1% Tween-20) for 2âh. nsp3 and nsp4 were probed using the primary and secondary antibodies indicated below, and developed with a chemiluminescent substrate (Thermo Fisher Science, 34095). Protein bands were visualized on the Bio-Rad ChemiDoc MP Imaging System (Bio-Rad). The following antibodies were used for western blots: rabbit polyclonal anti-SARS-CoV-2 nsp3 (Thermo Fisher Science, PA5-116947, dilution 1:5,000), rabbit polyclonal anti-SARS-CoV-2 nsp4 (Abclonal, A20281, dilution 1:1,000) and mouse monoclonal anti-GAPDH (Santa Cruz, sc-47724, dilution 1:5,000) were used as the primary antibodies. Anti-rabbit IgGâHRP antibody (Cell Signaling, 7074S, dilution 1:2,000) and anti-mouse IgGâHRP antibody (Cell Signaling, 7076S, dilution 1:2,000) were used as secondary antibodies.
SARS-CoV-2 nsp3ânsp4 DMV purification
Frozen HEK Expi293F cell pellets from 1âl cell culture were suspended in 80âml hypotonic buffer (20âmM HEPES (pH 7.5), 1.5âmM MgCl2 and 10âmM KCl) with protease inhibitor cocktail (Sigma-Aldrich, P8340) and GENIUS nuclease (ACROBiosystems, BEE-N3116). Cells were homogenized with a 50-ml Dounce homogenizer (Thomas Scientific) by repeated plunging for 40 strokes. The homogenate was then transferred to a beaker and sonicated in an ice bath with a probe sonicator (Branson Digital Sonifier SFX 550). The homogenate was disrupted for 15 cycles (2âs on and 4âs off) at 30% power. A total of 60 cycles were carried out with a 30-s delay between each 15 cycles. The homogenate was spun at 4,000g for 20âmin to remove the cell debris and the resulting supernatant was loaded to 2.5âml Strep-TactinXT 4Flow resin (IBA LifeSciences, 2-5010-025) pre-equilibrated with an equilibrate buffer (50âmM HEPES (pHâ7.5), 150âmM NaCl and 1âmM EDTA) for binding for 3âh in a gravity column. The resin was further washed with 10 column volumes of washing buffer (50âmM HEPES (pH 7.5), 500âmM NaCl and 1âmM EDTA) and then 10 column volumes of equilibrate buffer. The vesicles were eluted by elution buffer (50âmM HEPES (pHâ7.5), 150âmM NaCl, 1âmM EDTA and 50âmM biotin). For each elution step, the Strep-Tactin resin was incubated with elution buffer for 15âmin. All elution fractions were pooled together and loaded into a 13-ml ultracentrifugation tube and spun at 200,000g in a Thermo Scientific Sorvall WX ultracentrifuge with a TH-641 swing-bucket rotor for 1âh. The final pellet was gently suspended in STE buffer (10âmM Tris-HCl (pHâ8.0), 150âmM NaCl and 1âmM EDTA). All steps were finished within 1 day and samples were kept on ice or 4â°C throughout the purification procedure. Cryo-EM grids were prepared immediately after DMV resuspension.
Cryo-EM grid preparation for isolated DMVs
The 6-nm bovine serum albumin-coated gold fiducial beads (Aurion, 206.033) were concentrated 10 times by benchtop centrifugation for 30âmin at 4â°C. The gold beads were added to samples before freezing. A 3.5âµl volume of purified DMV samples was applied to freshly glow-discharged lacey carbon grids (Agar Scientific, AGS166-3, 300 mesh). The grids were blotted using a Vitrobot Mark IV with a blot force of 0 and blot time of 3.5âs with 95% humidity at 4â°C, and flash-frozen in liquid ethane before being stored in liquid nitrogen until data collection. Cryo-EM grids were screened in a 200-kV Thermo Scientific Glacios microscope with a Falcon 4 camera to optimize freezing conditions.
Cryo-ET data collection
The tilt series were acquired using a Thermo Fisher Krios equipped with a Falcon 4i camera and Selectris energy filter, with a slit width of 20âeV. PACE-tomo script31 within serialEM32 was used and a maximum of 15-μm imageâbeam shift was allowed when adding acquisition points. For purified DMV samples, a dose-symmetric scheme (group of 2) was used, with a tilt range of â51° to 51° (or â60° to 60°) at 3° increments and an exposure dose of 3 electrons per square Ã¥ngström per image at a magnification of Ã81,000 (pixel size: 1.571âà ). Tilt series were acquired with a defocus between â1âµm and â6âµm and a total of 5,170 tilt series were acquired. The tilt-series data were collected using multiple grids from two independent DMV purifications over multiple data collection sessions on the same microscope. The detailed data collection parameters are listed in Extended Data Table 1.
Subtomogram averaging and classification
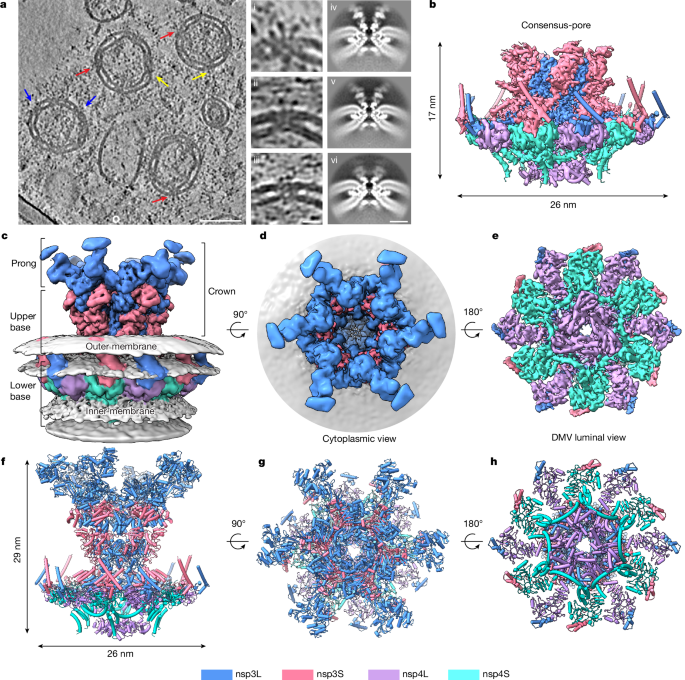
The raw micrographs in EER format were motion-corrected using relion_motioncor, applying gain reference correction. The blank images were removed by calculating the average image intensity using the clip command in IMOD. The motion-corrected images were then stacked into individual tilt series and aligned using batchruntomo, using the previous Python script (tomo_toolbox.py: https://github.com/ffyr2w/cet_toolbox). The resulting tilt series were imported to emClarity (v1.6.215) for contrast transfer function estimation, followed by particle picking with 8Ã-binned tomograms for template-matching (EMD-11514). The coordinates of picked particles and contrast transfer function information were exported to RELION (v4)33 for further 3D classification and 3D refinement. The initial 3D classification with C6 symmetry was carried out with 4Ã-binned pseudosubtomograms to remove the incorrectly picked particles. A total of 113,163 particles were obtained from the template-picked particles (598,699) and pulled together for another round of 3D classification into 6 classes. On average, about five nsp3ânsp4 complexes can be identified from each DMV after 3D classification, which is estimated from a subset of dataset containing 5,101 particles from 996 DMVs. The six classes were pooled into three main classes depending on the appearance of the cytoplasmic density of the pore complex and refined separately. The refinement was carried out sequentially with 4Ã-, 2Ã- and 1Ã-binned pseudosubtomograms with C6 symmetry, with three rounds of tomo frame alignment in total, which resulted in density maps of a mini-pore at 4.6âà , an extended-pore at 4.7âà and a full-length-pore at 6.2âà . Two classes (extended-pore and full-length-pore) were merged together to improve the cytoplasmic density, achieving a final resolution of the consensus full-length pore complex (full-pore) at 4.9âà . To improve the resolution of the core region of the pore complex, all of the particles were pooled together and refined as above, reaching a final resolution of 4.2âà in the consensus map (consensus-pore). Focused classification and refinement of the cytoplasmic crown in the full-length-pore was carried out to improve the density of the crown. Particle centres were shifted using relion_star_handler during focused classification and refinement. The subtomogram averaging and classification pipeline is summarized in Extended Data Fig. 2.
To resolve the density in the DMV lumen of the pore complex, we carried out focused classification of this region (skipping alignment) with either C3 or C1 symmetry, which resulted in classes with clear features of the nsp4-CTD, forming a trimer of nsp4-CTD dimer (Extended Data Fig. 3). The classes with discernible density of nsp4-CTD trimer of dimer were pooled together, and two classes were rotated by 180° along the zâaxis to merge into the other classes. The final map was further refined and reconstructed with C3 symmetry and sharpened with default b-factors, as determined by a Guinier plot. The local resolution was estimated in RELION (Extended Data Fig. 4).
Model building and validation
We first built the model using the C6-symmetry consensus-pore map at an overall 4.2âà resolution. The individual domains of nsp3 and nsp4 were predicted by Alphafold217 and cross-validated with the X-ray crystal structures (Supplementary Figs. 2 and 3). The ectodomains of the nsp3ânsp4 complex were predicted as a complex (Supplementary Fig. 4) and were placed into the density map manually together with other individual domains in Chimera. The predicted TMDs were manually fitted into the density maps, followed by manual real-space refinement in Coot34. The resulting structure was further refined by ISOLDE35 implemented in ChimeraX36. The final refinement enabling only rigid-body refinement and atomic displacement parameter (b-factor) refinement was conducted by phenix_refine37. The sequence register is validated by many bulky hydrophobic residues in the TMD (Extended Data Fig. 5c), and further checked using the checkMySequence tool38. The asymmetric unit of the consensus-pore complex contains four chains as followsânsp3L: 1411â1945 and nsp3S: 1403â1945; nsp4L and nsp4S: 31â401.
To model the C3-symmetry consensus-pore complex, the asymmetric unit refined from the C6-symmetry consensus-pore complex and Alphafold-predicted nsp4-CTD was manually fitted into the map. The structures were refined in ISOLDE and phenix_refine as above. To model the full-pore complex, we used the asymmetric unit from the C6-symmetry consensus-pore complex as the starting model and manually fitted the Alphafold-predicted DPUP-Ubl2-PLpro and CoV-Y domains into the map. The prong tip is in low resolution (12â15âà resolution) and the Mac2â3 and NAB domain were predicted as a complex using AlphaFold2 (Supplementary Fig. 5) to rigid-body fit into the map. The unresolved region between NAB and nsp3-TM1 consists of >200 amino acid residues, which is predicted to contain two helices, a βSM domain and a long-disordered loop (Supplementary Fig. 2d). The disordered loop (residues 1199â1241) can span a distance of 14ânm, and with the additional βSM domain (which is 4ânm in length), this unresolved region is sufficiently long to link NAB and nsp3L-TM1. The final structures were refined in ISOLDE and phenix_refine as above. Residues showing high clashing scores in phenix were manually corrected in Coot. The regions that are modelled in the full-pore map include the following: nsp3L: 417â1945; nsp3S: 1403â1945; nsp4L: 31â500; and nsp4S: 31â401. Most of the domains generated by Alphafold are similar to the final refined models except the TMDs. The statistics of Alphafold starting models, per-residue Q-scores using MapQ39 and overfitting assessment40 are presented in Extended Data Fig. 6 and Supplementary Figs. 6 and 7. The local-resolution-filtered maps are used for presentation in Chimera and ChimeraX. The structure alignment and comparison are carried out in Chimera using the matchmaker command.
Flow cytometry of transfected VeroE6 cells and cryo-EM grids preparation
VeroE6 cells were transfected with the wild-type or mutant pcDNA3.1-TwinStrep-EGFP-nsp3-nsp4 plasmids. At 24âh post-transfection, VeroE6 cells were washed with 1à PBS and treated with 0.25% trypsinâEDTA solution at 37â°C until cells were detached from the bottom of the 10-cm culture dish. Cells were pelleted at 200g for 3âmin and washed with 1à PBS, and then resuspended in 500âμl FACS buffer (1à PBS, 25âmM HEPES (pHâ7.5), 2% fetal bovine serum, 100âUâmlâ1 penicillin and 100âμgâmlâ1 streptomycin). The cell suspensions were filtered through 40-μm Falcon Cell Strainers (BD, 352340) before acquisition on a flow cytometer. Single-cell sorting and FACS analysis were carried out using a BD FACSAria SORP Cell Sorter with a 488-nm laser. Cells were considered positive when the fluorescence intensity was above a threshold value that was determined by the maximum intensity of the non-transfected control cells. Cells with GFP fluorescence signal were sorted and then seeded on the carbon side of the freshly prepared EM grids (Quantifoil Gold R2/2, 200 and 300 mesh) in 6-well plates. The grids were pre-treated as follows: glow-discharged for 45âs at 15âmA (PELCO easiGlow) and transferred, carbon side up, to a 6-well plate, then treated with 20âµgâmlâ1 of bovine plasma fibronectin (Sigma) in PBS for 30âmin and washed with PBS three times. Then the grids were UV-treated for 1âh. After the cells were seeded, the 6-well plates were incubated at 37â°C with 5% CO2 until plunge-freezing.
Plunge-freezing of cells grown on the EM grid
Grids were picked up from the 6-well plates and loaded into a Leica GP2 plunge freezer. The ethane temperature was set to â184â°C and the chamber was set to 37â°C with 80% humidity. An additional 3.5âμl of cell culture medium was applied to the back side of the grids, which were then blotted from the back for 7âs. The grids were immediately plunge-frozen in liquid ethane after blotting. Vitrified grids were stored in liquid nitrogen for further processing.
Cellular lamella preparation, cryo-ET data collection and tomogram reconstruction
Grids were clipped into cryogenic focused ion beam AutoGrids (Thermo Fisher Scientific). Lamella preparation was carried out on an Aquilos 2 cryogenic focused ion beam system (Thermo Fisher Scientific) using AutoTEM software. Grids were sputter-coated with platinum for 30âs, followed by 20âs of gas injection system coating. The gallium ion beam was gradually adjusted to lower values as the lamella thinning progressed (0.5ânA until 3âµm thick, 0.3ânA until 1.5âµm, 0.1ânA until 0.75âµm, then 50 pA until 300ânm and finally 30âpA until about 150â200ânm thickness).
The tilt series were acquired as videos on a Thermo Fisher Krios microscope operated at 300âkV with a Selectris energy filter using serialEM. The pre-tilt angle was estimated by comparing the medium-mag images of lamella acquired at ±45°. The tilt series were collected with a tilt range of â60° to 48° at 2° increments, a target defocus of â15âµm and an exposure dose of 1 electron per square Ã¥ngström per image at a magnification of Ã15,000 (pixel size: 8.571âà ). The raw micrographs in EER format were motion-corrected using relion_motioncor with gain reference applied. Dark images due to high tilt or neighbour ice contamination were removed by calculating the average image intensity using the clip command in IMOD. The motion-corrected images were then stacked into individual tilt series and aligned using AreTomo41. Tomograms were denoised using Topaz42 for better visualization.
Generation of recombinant SARS-CoV-2 with alterations
The infectious clone of SARS-CoV-2 on a BAC, named p-BAC-SARS-CoV-2, was generated and characterized as described previously22,43. The recombinant SARS-CoV-2 with nsp3 and nsp4 alterations was generated using homologous recombination. Briefly, two guide RNAs, sgRNA1 and sgRNA2 (Sangon), were used for CRISPRâCas9 cleavage of p-BAC-SARS-CoV-2 with the Cas9 enzyme digestion kit (NEB) according to the manufacturerâs instructions. The linearized p-BAC-SARS-CoV-2 was verified by gel electrophoresis and purified with a Gel Extraction Kit (Qiagen). The site-directed mutagenesis of nsp3 and nsp4 was carried out by overlapping PCR with primers F and R. The resulting gene fragments were further inserted into the linearized p-BAC-SARS-CoV-2 through homologous recombination using ClonExpress II One Step Cloning Kit (Vazyme). The primers are shown in Extended Data Table 2.
Recovery of recombinant viruses and immunofluorescence assay
BHK21-ACE244 cells at about 80% confluence in a six-well culture plate were transfected with 3âμg recombinant p-BAC-SARS-CoV-2 using Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific) according to the manufacturerâs instructions. At 6âh post-transfection, the cells were trypsinized and added as an overlay to infect VeroE6-TMPRSS244 cells in a six-well plate for 72âh. Cells were observed daily for the appearance of cytopathic effects. Also, the cell culture supernatant was collected at 24, 48 and 72âh post-transfection for detection of live virus titre and genome copies. A mutant virus without a steady increase of virus titre or copies was deemed as failed to be rescued.
Immunofluorescence assay was carried out to verify the rescue of recombinant virus. Cells were fixed with 4% paraformaldehyde in PBS at room temperature for 30âmin, and then the cell membrane was permeabilized with 0.2% (vol/vol) Triton X-100 in PBS for 5âmin. Cells were blocked in 4% bovine serum albumin buffer for 30âmin at 37â°C, washed with PBS, and incubated with the primary antibody (SARS-CoV-2 N polyclonal antibody45, 1:4,000) overnight at 4â°C, followed by washing with PBST for three times. The cells were then stained with Alexa Fluor 488 goat anti-rabbit IgG (Hâ+âL; Thermo Scientific, A-11064, 1:1,000) for 1âh at room temperature. After being washed with PBST, cells were visualized and imaged under a fluorescence microscope (Olympus).
Mass spectrometry
Protein samples were resolved using SDSâPAGE gel and visualized with Coomassie stain. The protein bands were cut into separate slices and subjected to in-gel digestion. Briefly, gel slices were subjected to reduction and alkylation by 10âmM TCEP and 55âmM 2-chloroacetamide, respectively. Protein digestion was carried out by incubating with trypsin (1ângâµlâ1) overnight at 37â°C. Subsequent tryptic peptides were extracted from the gel with 50% ACN/5% FA and 100% ACN sequentially. The peptide extracts were pooled together and SpeedVac dried. The peptides were desalted using C18 StageTips for analysis by liquid chromatography with tandem MS spectrometry (MS) analysis. Eluted peptides were analysed with a nanoelute UHPLC coupled to a Bruker timsTOF pro mass spectrometer. The peptide mixture was loaded onto an Aurora C18 UHPLC column (75âμm i.d.âÃâ25âcm lengthâÃâ1.6âμm particle size (IonOpticks)). Chromatographic separation was carried out using a linear gradient of 2â30% of buffer B (0.1% FA in ACN) at a flow rate of 300ânlâminâ1 over 27âmin. MS data were collected over an m/z range of 100 to 1,700. During MS/MS data collection, each thermal Ionization MS cycle was 1.1âs and included 1 MS plus an average of 10 PASEF MS/MS scans. Raw mass spectrometry data were processed using MaxQuant 1.6.14.0. Raw data were searched against the SARS-CoV-2 FASTA database containing 17 entries and the Human Swissprot FASTA database containing 20,361 entries, using the following settings: oxidized methionine and acetylation were selected as dynamic modifications, and carbamidomethyl as a fixed modification with a minimum peptide length of 7 amino acids was enabled. Confident proteins were identified using a targetâdecoy approach with a reversed database, a strict false-discovery rate of 1% for the peptide and peptide spectrum match level, and a minimum of â¥1 unique peptide and â¥2 peptide spectral matches.
Sequence conservation and pore radius analysis
The protein sequences were analysed using SnapGene Viewer (v7.1.1). The conservation plot was generated with WebLogo46 (https://weblogo.threeplusone.com/) using the protein sequences shown in Supplementary Fig. 9. HOLE was used for calculating the nsp3ânsp4 pore radius and central channel dimensions47.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.