Patient cohort
Patients diagnosed with CRC between 2004 and 2019, at Uppsala University Hospital or UmeÃ¥ University Hospital, were eligible for the study. Patients that had (1) a fresh-frozen biopsy or surgical specimen that was estimated by a pathologist to have a tumour cell content of â¥20%; and (2) a patient-matched source of control DNA from whole blood or fresh-frozen colorectal tissue stored in the biobank, were included. Clinical data were extracted from the national quality registry, the Swedish Colorectal Cancer Registry (SCRCR), and completed from medical records. The follow-up for alive patients was a minimum of 3.9âyears and a median of 8âyears (data lock 14 June 2023), with only one patient lost to follow-up and 994 (94%) with complete 5-year follow-up. Patients included with a diagnosis from 2010 (861 cases; 81%) were obtained from the Uppsala-UmeÃ¥ Comprehensive Cancer Consortium (U-CAN) biobank collections (Uppsala Biobank and Biobanken Norr)51. Unfixed tissue materials from tumour and healthy colon and rectum were handled on ice and frozen on the day of sampling or surgery52. Tissue collected in Uppsala was embedded in optimal cutting temperature (OCT) compound (Sakura) and stored at â70â°C. Tissue collected at UmeÃ¥ University Hospital was frozen in pieces and stored at â70â°C. Haematoxylin-and-eosin-stained sections from the frozen blocks were reviewed by a pathologist to confirm tumour histology and estimate tumour cell content. Matching healthy DNA samples were derived from peripheral blood (522 patients) or adjacent healthy tissue (541 patients). Control RNA was obtained from 120 patient-matched colon or rectum tissue samples. In total, tumours from 1,126 patients were sectioned and sequenced; however, 63 patients were excluded due to lack of high-quality DNA- or RNA-sequencing data from tumour or paired unaffected tissue.
Tissue retrieval and nucleic acid extraction
For tissue samples from Uppsala, five and eight cryosections of 10âµm each were used for RNA and DNA extraction, respectively. The DNA was extracted using the NucleoSpin Tissue kit (740952, Macherey-Nagel), and RNA was extracted using the RNeasy Mini Kit (74106, Qiagen). For tissue samples from UmeÃ¥, DNA and RNA were extracted using the AllPrep DNA/RNA/miRNA Universal kit (80224, Qiagen). Control DNA from blood samples was extracted using the NucleoSpin 96 Blood Core kit (740456, Macherey-Nagel) on a Genomics STARlet robot (Hamilton). For control samples derived from tissue, DNA and RNA were extracted using the same procedures as described for the tumour samples. DNA concentration was measured using the Qubit broad-range dsDNA assay kit in the Qubit system (Invitrogen), and RNA concentration and quality were assessed using the Bioanalyzer RNA 6000 Nano kit (Agilent) for samples from Uppsala and the Tape Station 2200 (Agilent) for samples from UmeÃ¥. RNA samples with RINââ¥â7, 28S:18S ratio â¥0.8 and concentration â¥60ângâµlâ1 were further analysed. We analysed bulk RNA from tumours and a smaller set of unaffected control CRC tissue to enable analyses across a large sample set. This approach, while common in such analyses, requires careful consideration of the impact of tissue heterogeneity on the results as systematic differences in cell type composition between CRC and healthy colorectal tissues could contribute to variations in gene expression profiles.
Whole-genome sequencing and data processing
The WGS libraries were constructed from 1,063 primary CRC tumours and their paired control samples according to the manufacturerâs instructions for the MGIEasy FS DNA Library Prep Set (1000006987, MGI). The libraries were sequenced on the DNBSEQ platform (MGI) and 100-bp paired-end sequencing was performed to yield data of â¥60à read coverage for all of the samples. During WGS data preprocessing, low-quality reads and adaptor sequences were removed by SOAPnuke (v.2.0.7)53 with the parameters â-l 5 -q 0.5 -n 0.1 –f AAGTCGGAGGCCAAGCGGTCTTAGGAAGACAA -r AAGTCGGATCGTAGCCATGTCGTTCTGTGAGCCAAGGAGTTGâ. Sentieon Genomics software (v.sentieon-genomics-202010; https://www.sentieon.com/) was used to map and process high-quality reads for downstream analysis54, which included the following optimised steps: (1) BWA-MEM (v.0.7.17-r1188) with the parameters â-M -K 100000000â in alt-aware mapping model was used to align each tumour and control sample to the human genome reference hg38 (containing all alternate contigs)55; (2) alignment reads were sorted by sort mode of Sentieon utility functions; (3) duplicate reads were marked by Picard (http://broadinstitute.github.io/picard/); (4) indel realignment and base quality score recalibration for aligned reads were carried out by GATK56; (5) and alignment quality control was done by Picard.
Somatic short-variant calling
Putative somatic SNVs, MNVs and/or indels were identified in each tumourâcontrol pair using multiple accelerated tools (TNhaplotyper, corresponding to MuTect257 of GATK3; TNhaplotyper2, corresponding to MuTect257 of GATK4; TNsnv, corresponding to MuTect58) and TNscope59 of Sentieon Genomics software (v.sentieon-genomics-202010.01). Passed somatic SNVs, MNVs and indels detected by at least two tools were retrained as ensemble somatic short variants for each paired controlâtumour sample. Allele depths of ensemble somatic short variants were recalculated by TNhaplotyper2 (v.sentieon-genomics-202010.01). High-confidence ensemble somatic short variants (depth of tumourââ¥â14, depth of controlââ¥â8, variant allele reads count of tumourââ¥â2, variant allele reads count of controlââ¤â2, variant allele fraction of tumourââ¥â0.005 and variant allele fraction of controlââ¤â0.02) were selected for downstream annotation and analysis. These variants were annotated with VEP cache v.101 (corresponding to GENCODE v.35) by Personal Cancer Genome Reporter (PCGR) (v.v0.9.1)60.
Somatic SVs and CNV
Somatic SVs were detected in each paired controlâtumour sample by BRASS (v.6.3.4; https://github.com/cancerit/BRASS) with the parameters â-j 4 –c 4 –s human –as GRCh38 –pr WGSâ, and ascatNgs61 (v.4.5; https://github.com/cancerit/ascatNgs) with the parameters â-g L -q 20 -rs âhumanâ -ra GRCh38 -pr WGS -c 4 -force -nobigwigâ. The genome cache file was generated by VAGrENT62 (v.3.7.0; https://github.com/cancerit/VAGrENT) with CCDS2Sequence.20180614.txt (https://ftp.ncbi.nlm.nih.gov/pub/CCDS/current_human/CCDS2Sequence.20180614.txt) and ensembl release-104 (http://ftp.ensembl.org/pub/release-104, Homo_sapiens.GRCh38.104.gff3.gz, Homo_sapiens.GRCh38.cdna.all.fa.gz, Homo_sapiens.GRCh38.ncrna.fa.gz). Other files for the required parameters of BRASS and ascatNgs were extracted from CNV_SV_ref_GRCh38_hla_decoy_ebv_brass6+.tar.gz (ftp://ftp.sanger.ac.uk/pub/cancer/dockstore/human/GRCh38_hla_decoy_ebv/CNV_SV_ref_GRCh38_hla_decoy_ebv_brass6+.tar.gz). The SVs present in control samples were filtered from the following analyses. Somatic CNVs were detected in each paired controlâtumour sample by facetsSuite (v.2.0.8; https://github.com/mskcc/facets-suite). An image of facetsSuite was pulled from docker://stevekm/facets-suite:2.0.8 and run with singularity (v.3.2.0)63. We used the aligned sequence BAM file as input data and executed FACETS in a two-pass mode with the default settings64. First, the purity model estimated the overall segmented copy-number profile, sample purity and ploidy. Subsequently, the dipLogR value inferred from diploid state in the purity model enabled the high-sensitivity model to detect more focal events. Allele-specific copy numbers for each high-confidence ensemble somatic short variant were annotated using the wrapper script âannotate-maf-wrapper.Râ with high-sensitivity output. The gene-level copy-number result was re-annotated with GENCODE v.35. Somatic copy-number states were grouped into eight classes based on total copy number (tcn) and minor copy number (also known as lower copy number; lcn) estimated by FACETS, including wild type class (one copy per allele; tcn=2, lcn=1), homozygous deletions (tcn=0, lcn=0), LOH (tcn=1, lcn=0), copy-neutral LOH (tcn=2, lcn=0), gain-LOH (tcn=3 or 4, lcn=0), gain (tcn=3 or 4, lcnâ¥1), amp-LOH (tcnâ¥5, lcn=0) and amp (tcnâ¥5, lcnâ¥1).
ecDNA detection
Amplicons were detected in each sample by PrepareAA (commit ba747ce; https://github.com/jluebeck/PrepareAA) with the parameters â–ref GRCh38 -t 4 –cngain 4.999999 –cnsize_min 50000 –downsample 10 –cnvkit_dir /home/programs/cnvkit.py –run_AAâ65,66. An image of PrepareAA was obtained from docker://jluebeck/prepareaa:latest and run with singularity (v.3.2.0). The amplicons were classified by AmpliconClassifier (v.0.4.4; https://github.com/jluebeck/AmpliconClassifier) with the parameters â–ref hg38 –plotstyle noplot –report_complexity –verbose_classification –annotate_cycles_fileâ67. The samples were classified on the basis of which amplicons were present in the sample as previously described24.
CIN signature quantification
The activities of the 17 CIN signatures presented previously68 were quantified using CINSignatureQuantification (v.1.0.0; https://github.com/markowetzlab/CINSignatureQuantification) with unrounded copy-number segments from facetsSuite. Tumours with normalized activities larger than zero, in any CIN signature, were identified as CIN samples.
MSI detection
The MSI status of CRC tumours was determined by running the MSIsensor2 (v.0.1, commit e0798c7; https://github.com/niu-lab/msisensor2) tumourâcontrol paired module (inherited from MSIsensor) with the parameters â-c 15 -b 4â. MSIsensor2 automatically detects somatic homopolymers and microsatellite changes and calculates the MSI score as the percentage of MSI-positive sites in all valid sites. MSIsensor2 software comprises of two modules: tumour-only and paired. The tumour-only module is an algorithm for tumour-only sequencing data, with a recommended cut-off score of 20. By contrast, the paired module is derived from the original MSIsensor1 and the recommended threshold score is 3.5 for MSI69. Correlation analyses between the two modules showed a strong correlation between their results, so we selected the paired module. Furthermore, some studies subdivide MSI samples into MSI-low (scores between 3.5 and 10) and MSI-high (scores above 10) based on the paired module. However, our analysis revealed that most of the samples with scores in the MSI-low range according to the paired module had scores above 20 in the tumour-only module, so we considered all samples with an MSI score of â¥3.5 as having MSI.
Identification of significantly mutated genes
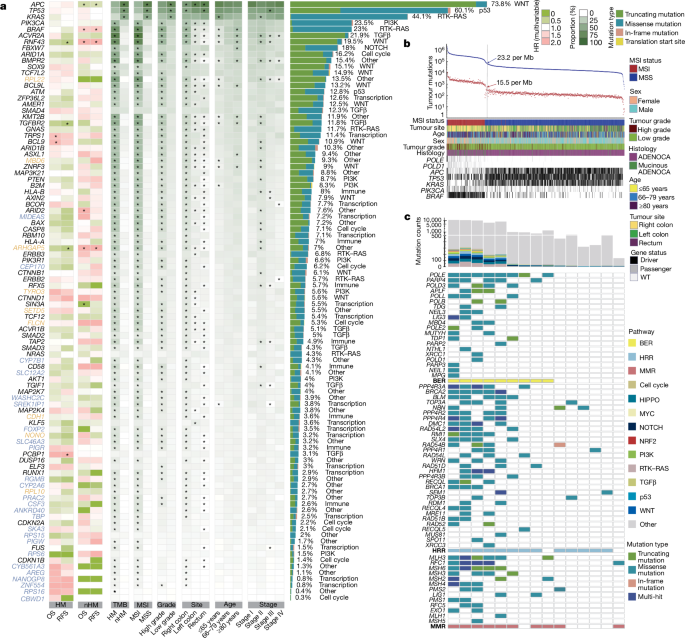
HM tumours associated with MSI or POLE mutation are frequently found in CRC. To avoid signals from samples with lower mutation burden from being masked during downstream WGS analyses, we first classified the tumours as HM or nHM based on the total count of somatic short variants according as previously described70:
$${N}_{{\rm{SNV}}} > {N}_{{\rm{median}}\_{\rm{SNV}}}+1.5\,\times \,\text{interquartile range}$$
After a first round of calculations, each HM sample was split into two separate artificial samples with an equal number of mutation counts. This process was repeated until no HM samples were detected by the formula. Outlier times indicate how many times a sample was called as HM in this process. The mutational heterogeneity caused by the increased mutation burden of HM tumours can reduce the power to detect driver genes and affect the identification of mutational signatures4,27,71. To identify CRC driver genes, we ran dNdScv72 (v.0.1.0, commit dcbf8e5; https://github.com/im3sanger/dndscv) on the whole cohort and on HM and nHM samples separately. A list of known cancer genes to be excluded from the indel background model was compiled from the COSMIC Cancer Gene Census73 (v.95) and intOGen Compendium Cancer Genes (release date 1 February 2020, https://www.intogen.org/)72,74,75,76,77,78,79,80. Covariates (a matrix of covariates (columns) for each gene (rows)) were updated to covariates_hg19_hg38_epigenome_pcawg.rda (commit 9a59b89; https://github.com/im3sanger/dndscv_data). The reference database was updated to RefCDS_human_GRCh38_GencodeV18_recommended.rda (commit 9a59b89; https://github.com/im3sanger/dndscv_data). The dNdScv R package includes two different dN/dS-based algorithms, dNdSloc and dNdScv. dNdSloc is similar to a traditional dN/dS implementation, while dNdScv also takes into account variable mutation rates across genes and adds a negative binomial regression model using epigenomic covariates to infer the background mutation rate. The list of significant genes was selected by BenjaminiâHochberg-adjusted P values (qall_loc<0.1 or qglobal_cv<0.1) and merged from both dNdSloc and dNdScv. Long genes81, olfactory receptor genes and genes with transcript per million (TPM)â>â1 in less than ten tumours were excluded from the potential driver gene list. Mutually exclusive or co-occurring sets of driver genes were detected using the modified somaticInteractions function of Maftools82 (v.2.12.0), which performs pair-wise Fisherâs exact tests to detect significant (BenjaminiâHochberg false-discovery rate (FDR)â<â0.1) pairs of genes.
Identification of broad and focal somatic copy-number variation
To determine significantly recurrent broad and focal somatic CNVs, GISTIC2.083 (v.2.0.23) was run on resulting segmentation profiles from facetsSuite high-sensitivity models with the parameters â-ta 0.3 -td 0.3 -qvt 0.25 -rx 0 -brlen 0.7 -conf 0.99 -js 4 -maxseg 25000 -genegistic 1 -broad 1 -twoside 1 -armpeel 1 -savegene 1 -gcm extreme -smallmem 1 -v 30â. A higher-amplitude threshold according to GISTIC was used for focal copy-number-alteration classification, tumour and control log2 ratioâ>â0.9 for amplifications and <â0.3 for deletions83. Recurrently amplified or deleted regions were identified by GISTIC peaks and genes within each peak were summarized for further analyses.
Mutational signature analysis
Analyses of mutational signatures were performed by SigProfilerExtraction84 (v.1.1.4) with the parameters â–reference_genome GRCh38 –opportunity_genome GRCh38 –minimum_signatures 1 –maximum_signatures 40 –nmf_replicates 500 –cpu 12 –gpu True –cosmic_version 3.2â. SigProfilerExtraction consists of two processes: de novo signature extraction and signature assignment27,85,86. Hierarchical de novo extraction of SBS, DBS and ID signatures from all samples was followed by estimation of the optimal solution (number of signatures) based on the stability and accuracy of all 40 solutions. After signatures were identified, the activities of each signature were estimated by assigning the number of mutations in each extracted mutational signature to each sample. SigProfilerExtraction also decomposed de novo signatures to the COSMIC16 signature database27 (v.3.2). The cosine similarity87 between mutational signatures of this and the GEL cohorts28, and this and the PCAWG cohorts27 (COSMIC v.3.3), were calculated using R (v.4.2.0). A de novo signature was considered novel if the cosine similarity to both GEL and PCAWG signatures was <0.85. The mutational signature associations between decomposed signatures were calculated by Stats::cor (method = âspearmanâ) and corrplot::cor_mtest (conf.level = 0.95, âspearmanâ) in R (v.4.2.0), and those with an FDR-adjusted Pâ<â0.05 were considered to be statistically significant88.
Analyses of non-coding somatic drivers in regulatory elements
Regulatory elements were defined using SCREEN (Registry of cCREs V3; https://screen.encodeproject.org/), a registry of cCREs derived from ENCODE data89. Active cCREs annotated in 13 tissue samples (small intestine, transverse, sigmoid, left colon tissues) and 7 cell lines (CACO-2, HCT116, HT-29, LoVo, RKO, SW480 and HCEC 1CT) derived from colon were collected and downloaded from SCREEN, where cCREs are classified into six active groups (promoter-like signatures (PLS), proximal enhancer-like signatures (pELS), distal enhancer-like signatures (dELS), DNase-H3K4me3, CTCF-only and DNase-only) based on integrated DNase, H3K4me3, H3K27ac and CTCF data. Furthermore, the list of genes possibly linked to a cCRE according to experimental evidence (for example, Hi-C) was extracted from the cCRE Details page of the website. Driver analyses were performed by ActiveDriverWGS71,90 (v.1.1.2, commit 351ca77; https://github.com/reimandlab/ActiveDriverWGSR) with the parameters â-mc 4 -rg hg38 -fh 300â on non-HM samples for each cCREs groups. The missense mutations in the analyses of regulatory regions were removed to avoid confounding signals from known cancer drivers. Mutated elements with a BenjaminiâHochberg FDRâ<â0.05 were considered to be significant and were used in the following analyses90. To evaluate the functional effects of driver cCREs, we examined their prognostic value and compared the expression levels of their linked genes. Cox proportional hazard analyses were performed to identify prognosis-associated cCREs using the Survival R package (v.3.3-1). Furthermore, potential associations between each cCRE and the expression levels of their linked genes were analysed by comparing raw expression values between groups of mutated and wild-type samples using two-sided Wilcoxon rank-sum tests. An FDR adjustment was applied to the P values from the Wilcoxon test and genes with FDR-adjusted Pâ<â0.05 were considered to be differentially expressed with statistical significance. Finally, cCREs that had an impact on the expression of linked genes were analysed according to survival.
Mitochondrial genome somatic mutation and copy-number estimation
We used multiple tools in the GATK4 (v.4.2.0.0) workflow to extract reads mapped to the mitochondrial genome from WGS, perform the mtDNA variant calling and filter the output VCF file based on specific parameters, according to GATK best practices (https://gatk.broadinstitute.org/hc/en-us/articles/4403870837275-Mitochondrial-short-variant-discovery-SNVs-Indels-). Furthermore, false-positive calls potentially caused by reads of mtDNA into the nuclear genome (NuMTs) were examined. These mutations normally have a low VAF but are highly recurrent in multiple tumours, as well as in matched control samples. To remove these false positives, we used stringent sample filtering, especially on variants with heteroplasmy <10%. We first performed two statistical tests as previously described30: (1) the VAF of a mutation in the matched control sequences needed to be <0.0034; and (2) the ratios of:
$${N}_{{\rm{M}}{\rm{u}}{\rm{t}}{\rm{C}}{\rm{t}}{\rm{r}}{\rm{l}}}/{{\rm{R}}{\rm{D}}}_{{\rm{C}}{\rm{t}}{\rm{r}}{\rm{l}}}/({N}_{{\rm{M}}{\rm{u}}{\rm{t}}{\rm{C}}{\rm{t}}{\rm{r}}{\rm{l}}}/{{\rm{R}}{\rm{D}}}_{{\rm{C}}{\rm{t}}{\rm{r}}{\rm{l}}}+{N}_{{\rm{M}}{\rm{u}}{\rm{t}}{\rm{T}}{\rm{u}}{\rm{m}}}/{{\rm{R}}{\rm{D}}}_{{\rm{T}}{\rm{u}}{\rm{m}}})$$
needed to be <0.0629, where NMut refers to mutation allele count, RD to average read depth, and Ctrl and Tum are control and matched tumour tissues, respectively. These cut-offs were adapted from a previous study30 and set by the median results of all mutation candidates plus 2 times the interquartile range. As the mutation rate of tumour-specific NuMTs is around 2.3% (ref. 91), we retained mutations with a frequency of <0.023. To avoid false-negative calls, mutations with VAFmaxâ<â0.1 and VAFmedianâ<â0.05 were examined, and the tumours in which the mutation had VAFâ>â0.05 were retained92. The mean sequencing depth for the mitochondrial genome was 14,286-fold, allowing high-sensitivity detection of somatic mutations at a very low levels of heteroplasmy; thus, variants with 0.01â<âVAFâ<â0.95 were used for subsequent analyses. For mtDNA copy-number calculation, we used pysam (v.0.15.3) to filter and estimate the raw copy number of each sample. We then calculated the normalized copy number as described previously5. The survival best cut-point of mtDNA copy number was identified with surv_cutpoint (maxstat test: Maximally Selected Rank and Statistics) implemented in survminer (v.0.4.9). The associations between mutational signatures and mtDNA copy number were calculated by Stats::cor (method = âspearmanâ) and corrplot::cor_mtest (conf.level = 0.95, âspearmanâ) in R (v.4.2.0), and those with FDR Pâ<â0.05 were considered to be statistically significant88.
Relative timing of somatic variants and copy-number events
For each nHM tumour, allele-specific copy-number-annotated high-confidence ensemble somatic short variants and high-sensitivity copy-number events of autosomes (except the acrocentric chromosome arms 13p, 14p, 15p, 21p and 22p) were timed and related to one another with different probabilities using PhylogicNDT25,93 (v.1.0, commit 84d3dd2; https://github.com/broadinstitute/PhylogicNDT). Single-patient timing and event timing in the cohort were inferred using PhylogicNDT LeagueModel as previously described26. The driver gene list identified in this cohort was specified to run PhylogicNDT.
RNA sequencing and determination of gene expression levels
The rRNA was removed from total RNA using the MGIEasy rRNA Depletion Kit (1000005953, MGI) and sequencing libraries were prepared for the 1,063 primary CRC tumours and 120 adjacent control tissue samples using the MGIEasy RNA Library Prep Kit V3.0 (1000006384, MGI) according to the manufacturerâs instructions. Sequencing of 2âÃâ100âbp pairedâend reads was performed using the DNBSEQ platform (MGI) with a target depth of 30âmillion paired-end reads per sample. Pre-processing of RNA-seq data, including removal of low-quality reads and rRNA reads, was performed using Bowtie2 (v.2.3.4.1)94 and SOAPnuke. Clean sequencing data were mapped to human reference GRCh38 using STAR (v.2.7.1a)95. Expression levels of genes and transcripts were quantified using RNA-SeQC (v.2.3.6)96. Transcripts with expression level 0 in all samples were excluded from further analyses and the mRNA expression matrix (19,765âÃâ1,183) was converted to log2(TPMâ+â1).
Detection of oncogenic RNA fusions
Gene fusions were detected by STAR-Fusion97 (v.1.10.0; https://github.com/STAR-Fusion/STAR-Fusion) using clean FASTQ files with the parameters â–FusionInspector validate –examine_coding_effect –denovo_reconstruct –CPU 8 –STAR_SortedByCoordinateâ and Arriba98 (v.2.1.0; https://github.com/suhrig/arriba) starting with BAM files aligned by STAR95 (v.2.7.8a; https://github.com/alexdobin/STAR). An image of STAR-Fusion was pulled from docker://trinityctat/starfusion:1.10.0 and run with singularity (v.3.2.0). Genome lib used in STAR-Fusion was downloaded from CTAT genome lib (https://data.broadinstitute.org/Trinity/CTAT_RESOURCE_LIB/__genome_libs_StarFv1.10/GRCh38_gencode_v37_CTAT_lib_Mar012021.plug-n-play.tar.gz). Aligned BAM files for Arriba were generated as described in the user manual (https://arriba.readthedocs.io/en/latest/). Gene fusions from Arriba were then annotated by FusionAnnotator (v.0.2.0; https://github.com/FusionAnnotator/FusionAnnotator) and merged with results of STAR-Fusion. Merged results were then filtered and prioritized with putative oncogenic fusions by annoFuse99 (v.0.91.0; https://github.com/d3b-center/annoFuse).
Unsupervised expression classification for generation of CRPS
We used Seurat (v.4.1.0) to identify stable clusters of all CRC samples and among MSI tumours100. Potential batch effects or source differences between samples were corrected by Celligner101 (v.1.0.1; https://github.com/broadinstitute/Celligner_ms), and the resulting matrix was imported into Seurat as scale data. Three different parameters were evaluated by repeating clustering with different k.param in FindNeighbors (10 to 30, step=5), number of principle components (10 to 100, step=5) and resolution in FindClusters (0.5 to 1.4, step=0.1). The stability of clusters was assessed by Jaccard similarity index and the preferred clustering result (resolution=0.9, PCâ=â20, Kâ=â20) was determined by scclusteval102 (v.0.0.0.9000).
CMS and iCMS classification
For the CMS classification, three CMS classifier algorithms (CMSclassifier (v.1.0.0) with random-forest prediction41, CMSclassifier-single sample prediction41 and CMScaller103 (v.0.9.2)) were evaluated and the results from the CMSclassifier-random forest was used. Expression data were processed using these three R packages separately or combined, generating four sets of results. In the combined mode, the CMS subtype of each tumour was determined when two algorithms made the same prediction, otherwise it was assigned as NA. Among all four sets of results, CMSclassifier-random forest predicted the most control samples as NA and assigned more MSI samples to CMS1, indicating a lower false-positive rate and a higher accuracy. The Intrinsic CMS (iCMS) classification was performed based on 715 marker genes of intrinsic epithelial cancer signature as described previously7. The iCMS2 marker genes were obtained from the iCMS2_up and iCMS3_down lists, and the iCMS3_up and iCMS2_down lists were used as iCMS3 markers. Subsequently, the iCMS2 and iCMS3 scores for each tumour were calculated using the ântpâ function of the CMScaller R package. Tumours were defined as indeterminate if permutation-based FDR was ⥠0.05.
Model building and validation of CRPS classification
To validate the CRPS de novo classification, we built a classification model based on a deep residual learning framework, involving the following steps: (1) gene expression data were first converted into pathway profiles by single-sample gene set enrichment analysis (ssGSEA104) implemented in Gene Set Variation Analysis (GSVA105 (v.1.42.0), parameters âmin.sz=5, max.sz=300â) using MSigDB106,107,108 (v.7.4). We eventually obtained 30,049 pathways for 1,183 samples, including 1,063 tumours and 120 adjacent unaffected control samples. (2) RelieF implemented in scikit-rebate109 (v.0.62) was used to refine the obtained pathway features. The RelieF algorithm used nearest-neighbour instances to calculate feature weights and assigned a score for the contribution of each feature to the CRPS classification. The features were then ranked by scores and the top 2,000 were selected for the model training. (3) We used TensorFlow110 (v.2.3.1) to construct the supervised machine learning model with a 50-layer residual network architecture (ResNet50-1D), of which the 4 stacked blocks were composed of 48 convolutional layers, 1 max pool and 1 average pool layer. The filters and strides were set as previously described111 and the kernel size was set to height. The activation function was set to SeLU, except for the last layer, which used Softmax for full connection. During model compilation, we used the Nadam algorithm as the optimizer in terms of speed of model training and chose Categorical Crossentropy as loss of function in the classification task. To train the model sufficiently, epochs were set to 500 and LearningRateScheduler in TensorFlow was used to control the learning rate precisely in the beginning of each epoch; finally, ModelCheckpoint in TensorFlow was used to save the model with the maximum F1 score. (4) All 1,183 samples were divided into a training set (80%), a test set (10%) and a validation set (10%). Before the model training, a 1D vector, which represents each gene sets row of samples (gs1,âgs2,ââ¦,âgsn), was converted to a 2D matrix (1,ânfeatures) with the np.reshape function, and used as the input data for Tensor (input shape structures were set to (none, â1, 2000)). ResNet50 learned the representations of the input data and was fitted to the training set. The number of output classes in TensorFlow was set to 6, corresponding to 5 clusters of CRPS and a normal sample cluster. To avoid bias caused by class imbalance during the learning process, the Random OverSampling Examples algorithm in Imbalanced-learn112 (v.0.9.0) was applied to ensure that at least one sample from each CRPS class could be randomly selected for model training. Samples with class probabilities of less than 0.5 were categorized as NA. Moreover, Shapley Additive exPlanations (SHAP)113 was applied to explain the model predictions on CRPS classifications, the molecular features of which could therefore be interpreted. To test the CRPS classification model, a total of ten external CRC datasets (nâ=â2,832) from NCBI GEO114 (GSE2109, GSE13067, GSE13294, GSE14333, GSE20916, GSE33113, GSE35896 and GSE39582), NCI Genomic Data Commons115 (TCGA-COAD4, TCGA-READ4) and AC-ICAM31 were uniformly processed and transformed to pathway profiles with ssGSEA. After class prediction of these CRC samples by our CRPS classification model, survival and pathway analyses were performed. Among these external datasets, only the GSE39582, TCGA and AC-ICAM cohorts have sufficient sample sizes and completeness of clinical data to allow survival analyses. Thus, the comparisons of prognostic prediction between CMS, iCMS and CRPS were performed using these three datasets individually and combined. Pathway analyses of CRPS from our dataset and from TCGA were performed using CMScaller103. The CRPS classification model is available at GitHub (https://github.com/SkymayBlue/U-CAN_CRPS_Model).
Pathway analyses
GSEA106 (v.4.2.3 desktop) and MSigDB107,108 (v.7.4) were used in pathway analyses, with the following settings: filter âgeneset min=15 max=200â. We also used PROGENy116 (v.1.16.0) to investigate 14 oncogenic pathways in CRPS, as previously described. The integrated presentation of pathways regulated by CRC somatic alterations were processed using PathwayMapper (v.2.3.0; http://pathwaymapper.org/)117. Pathway templates were merged, including cross-pathway interactions118, using the Newt tool (v.3.0.5; https://newteditor.org/)119, which allows experimental data to be visually overlaid on the pathway templates.
Hypoxia scoring and associations with mutational features
Hypoxia scores were calculated for 1,063 CRC tumours and 120 unaffected control samples using the Buffa hypoxia signature45 as previously described44. In brief, samples with an mRNA abundance above the median tumour value of each gene in the signature were given a Buffa hypoxia score of +1, otherwise they were given a Buffa hypoxia score of â1. The sum of the score for every gene in the signature is the hypoxia score of the sample. We used a linear model to analyse the associations between hypoxia scores and mutational features of interest in all tumours, nHM tumours and HM tumours using R stats package (v.4.1.0). For each mutational feature tested in the cohort, a full model and a null model were created and both were adjusted for tumour purity, age at diagnosis and sex120. The equations for the two models were adapted from a previous study44:
$${\rm{Full}}={\rm{hypoxia}} \sim {\rm{feature}}+{\rm{age}}+{\rm{sex}}+{\rm{purity}}$$
$${\rm{Null}}={\rm{hypoxia}} \sim {\rm{age}}+{\rm{sex}}+{\rm{purity}}$$
Comparisons between the two models were made using ANOVA, and hypoxia was considered to be statistically significantly associated with a mutational feature when FDR- or Bonferroni-adjusted P values were <0.1. Bonferroni adjustment was applied only to P values when <20 tests were conducted. The scaled residuals for all full models were calculated using the simulateResiduals function in the DHARMa package121 (v.0.4.5), and their uniform distributions were verified using the KolmogorovâSmirnov test. Tested mutational features included mutational signatures, SNV, CNV and SV densities, driver mutations and subclonality. In the mutational signature analysis, the proportion of each signature in each tumour was used in the full model. To test the association between hypoxia and specific genetic alterations, we considered 22 metrics of mutational density, including 10 SNV mutation counts encompassing all regions, coding region, non-coding region, nonsynonymous, SNV, DNV, TNV, DEL, INS and INDEL; 8 metrics of CNV mutational density which were adapted from PCAWG44, including the fraction of genome with total copy-number aberrations (PGA, total), PGA gain, PGA loss, PGA gain:loss, average CNV length, average CNV length gain, average CNV length loss and average CNV length gain:loss; and 4 SV types, including deletion, inversion, tandem-duplication and translocation. Mutational density by deciles of all 22 metrics were calculated using the R package dplyr122. Finally, in the subclonality analysis, clonal and subclonal mutations and numbers of subclones for each tumour were derived from PhylogicNDT as described above.
Prediction of cell types in the tumour microenvironment
The CIBERSORT48 (v.1.04) and xCell47 (v.1.1.0) computational methods were applied with the default settings on TPM gene expression data for microenvironment estimation.
Survival analyses
The OS was defined as time from diagnosis of primary tumour to death or censored if alive at last follow-up, RFS was defined as time from surgery to earliest local or distant recurrence date or death, or censored if no recurrence or death at last follow-up, while survival after recurrence was defined as the time from recurrence to death. The OS analyses included all patients with stage IâIV, whereas patients with stage IV at diagnosis were excluded in the RFS analyses. Separate OS analyses were also performed for stage IâIII for some variables. Coxâs proportional hazards models were built to determine the prognostic impact of clinical and genomic features using the R packages finalfit and survival (v.1.0.4/v3.3-1). Univariable Cox regression was performed on all identified coding or non-coding drivers and clinical variables, while multivariable Cox regression was applied to drivers that were statistically significant in the univariable analyses (Pâ<â0.05) with co-variates including tumour site, pretreatment status, tumour stage, age groups, tumour grade and hypermutation status. The OS and RFS curves were constructed using the KaplanâMeier method and the differences between groups were assessed using the log-rank test, using the R package survminer (v.0.4.9). In the Supplementary Tables 18, 19, 21, 23 and 30 showing associations with either OS or RFS, analyses showing Pâ<â0.05 were marked in bold. No compensation for multiple testing was done in these analyses.
Ethics declarations
Patient inclusion, sampling and analyses were performed under the ethical permits 2004-M281, 2010-198, 2007-116, 2012-224, 2015-419, 2018-490 (Uppsala EPN), 2016-219 (Umeå EPN) and the Swedish Ethical Review Authority 2019-566. All of the participants provided written informed consent at enrolment. All of the samples were stored in the respective central biobank service facilities in Uppsala (Uppsala Biobank) and Umeå (Biobanken Norr) and obtained for use in analyses here after approved applications. Sequencing and sequence data analyses of pseudonymized samples were performed at BGI Research, which had access to patient age range, sex and tumour-level data. Samples and data were transferred from UU to BGI Research under Biobank Sweden MTA and applicable GDPR standard terms for transfer to third countries. The analysis of patient-level data was performed at UU. The study conformed to the ethical principles for medical research involving human participants outlined in the Declaration of Helsinki.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.