Cell culture in two-dimensional monolayers
HEK293 and MDCK-II (00062107, Public Health England) cells were cultured as two-dimensional monolayers in minimal essential medium with 5% fetal bovine serum, 1% non-essential amino acids, 1% sodium pyruvate, and 1% GlutaMAX without addition of antibiotics at 37â°C with 5% CO2. Two-dimensional monolayers on transgenic cell lines were selected in the presence of geneticin (400âμgâmlâ1). Transient transfection of MDCK-II cells was carried out using Lipofectamine 2000 after cells reached a confluency of 70%. Transgenic cell lines were created from synthesized genes cloned using Not-I and Asc-I cutting sites into mammalian expression plasmids designed in-house (pOCC series) with N-terminal Dendra2, a selection marker against neomycin-geneticin and a CMV promotor. To obtain stable lines, single clone selection was carried out by fluorescence-activated cell sorting.
For seeding of two-dimensional monolayers, 400âµl of cell suspension (0.5 million cells per millilitre) was transferred on to the external side of a standing transwell filter (Corning 3460). Cells were left to adhere at 37â°C in a sterile environment for 30âmin. Afterwards, each transwell filter was mounted in a one-well plate with 1âml of culture medium on the bottom side and 500âµl of medium on the internal side.
Generation of three-dimensional cysts and transepithelial permeability assay
For the adherent three-dimensional cell culture cysts, MDCK-II cells were resuspended from a confluent monolayer into a single-cell suspension. The surface of a Mateck dish (35âmm glass bottom, P35G-0.170-14-C) was coated with a solution of laminin (0.5âmgâmlâ1) for 1âh at 37â°C, 5% CO2. Afterwards, a suspension of 20,000 cells were seeded on the coated surface in the respective culture medium complemented with 5% Matrigel on ice. Cells were cultured for 5â6 days until reaching 30â40âµm in diameter. To measure the permeability of dextran (Alexa647, 10k) cyst medium was supplemented with 10âμM dextran. After 15âmin of incubation, cysts were imaged using a confocal microscope to evaluate the distribution of dextran. The permeability of dextran into the lumen was quantified by measuring the intensity in the lumen and dividing this by the average intensity outside the cysts.
CRISPRâCas9 knock-in MDCK-II cells
CRISPRâCas9 technology was used to generate several single and double knock-in MDCK-II cell lines. ZO-1âmNeon was generated by fusing a copy of mNeon at the N terminus of the initial exon of endogenous ZO-1. Using the cell line expressing this fusion construct as a background, we tagged several other scaffold proteins with mScarlet at the N terminus of the initial exon of endogenous PATJ or ZO-2 and at the carboxyl terminus of MAGI-3 to generate double knock-in lines. Briefly, specific CRISPR RNA (crRNA, Integrated DNA Technologies (IDT) lt-R CRISPRâCas9 crRNA) for each gene of interest was designed using online tools Crispor (http://crispor.tefor.net) and ChopChop (https://chopchop.cbu.uib.no). Trans-activating crRNA (IDT catalogue no. 1072532) and crRNA were annealed at a ratio of 1:1 by incubation for 5âmin at 95â°C and then 10âmin at room temperature to generate guide RNA (gRNA). All gRNAs can be found in Supplementary Table 4. Next, the donor plasmid containing 5â² and 3â² homology arms was synthesized in a pUC57 Kan (Genescript) or a pUCIDT Kan (IDT) (Supplementary Table 4). A PTisy plasmid containing the fluorescence tag (mSc or mNn) was integrated into the donor plasmid through digestion using restriction enzymes. Afterwards, a ribonucleoprotein complex was assembled by mixing 1âμl (10âμgâμlâ1) of the recombinant Alt-R S.p. HiFi Cas9 nuclease (IDT, catalogue no. 1081060) and 1âμl of gRNA (100âμM) with the reaction buffer, followed by incubation at room temperature for 20âmin. The ribonucleoprotein complex and 1âμg of the donor plasmid were cotransfected through electroporation and overlapped with the exon sides. Electroporation of each complex was performed in 300,000 cells (Invitrogen NEON electroporation machine and kit, two pulses, 20âms, 1,200âV). Cells were then plated in growth medium in a six-well plate. Medium was exchanged after 24âh. Cells were sorted 48â72âh after electroporation. Fluorescent cells (mNn or mSc) were enriched (one or two cycles), and single clones were plated in one well of a 96-well plate. To validate genetic modification, we first did a PCR amplification. The correct insertion of the fluorescent tag at the correct locus of the gene of interest was verified by sequencing genotyping. Sequencing confirmed homozygous insertion of the tags, and imaging confirmed that mS tagging of the endogenous proteins resulted in proper colocalization with mN-ZO-1 at the tight-junction belt in confluent monolayers.
CRISPRâCas9 knockout in MDCK-II cells
For deletion of PATJ âexon 3, we used two gRNAs on each site of the targeting exon for each gene, selected on the basis of low off-target activity using http://crispor.tefor.net. The gRNAs were ordered as crRNAs from IDT. gRNAs were transfected in pairs, and cell pools were tested for deletion events by PCR using primers spanning the targeting exon. Best-performing gRNA pairs were used for cell cloning. Single cells were screened for the deletion by PCR using primers spanning the targeting exon. Flanking PCR with one primer outside and one primer inside the deletion was performed on the knockout candidate clones to verify the absence of the WT allele. Deletion alleles were verified by Sanger sequencing.
Sequencing of two clones confirmed deletion of the first exon, including a frame shift leading to an early stop codon. Western blots and quantitative PCR (qPCR) against the N-terminal L27 domain of PATJ, encoded by the first exon, confirmed deletion of this domain. Immunostaining of the more C-terminal PDZ4 confirmed that the remaining truncated protein was located in the cytosol and was not present at the tight junction. qPCR was performed using a Qiagen RNeasy (74104) mini kit after bioanalysis showed that the primers were of good quality (Supplementary Table 4).
Two-colour super-resolution STED microscopy
STED imaging was performed with a commercial confocal infinty-line STED microscope (Abberior Instruments) operated using the Imspector software (v.16.2.8415), with pulsed laser excitation (490ânm, 560ânm, 640ânm, 40âMHz), and Ã60 water and Ã100 oil objectives (Olympus). Star Orange was imaged with a pulsed laser at 560ânm, and excitation of Abberior Star Red was performed at 640ânm. The depletion laser for both colours was a 775ânm, 40âMHz, pulsed laser (Katana HP, 3âW, 1âns pulse duration, NKT Photonics). The optimal combination of excitation intensity, STED power and pixel dwell time were established by minimizing the onset of strong photobleaching. To reduce high-frequency noise, STED images were filtered with a two- or three-dimensional Gaussian with a sigma of 0.8 pixels. Quantification and segmentation of STED data was done using custom software written in MATLAB52.
Live imaging microscopy
Live imaging kinetics were acquired using a Wide-Field Delta Vision Elite system equipped with an Olympus PlanApo N Ã60/1.42 objective, SSI Lumencor illumination system and Roper Evolve EMCCD camera. Perturbation experiments were acquired with a wide-field General Electric Delta Vision system equipped with an Olympus PlanApo N Ã60/1.42 objective, and mN and mCh were excited with an Applied Precision Xenon Arc Lamp (V300-Y18). High-resolution imaging was done with a Yokogawa Spinning Disk Field Scanning Confocal System (CSU-X1 Nikon, Andor iXOn Ultra) using a Ã60 water objective.
MDCK-II calcium switch assay
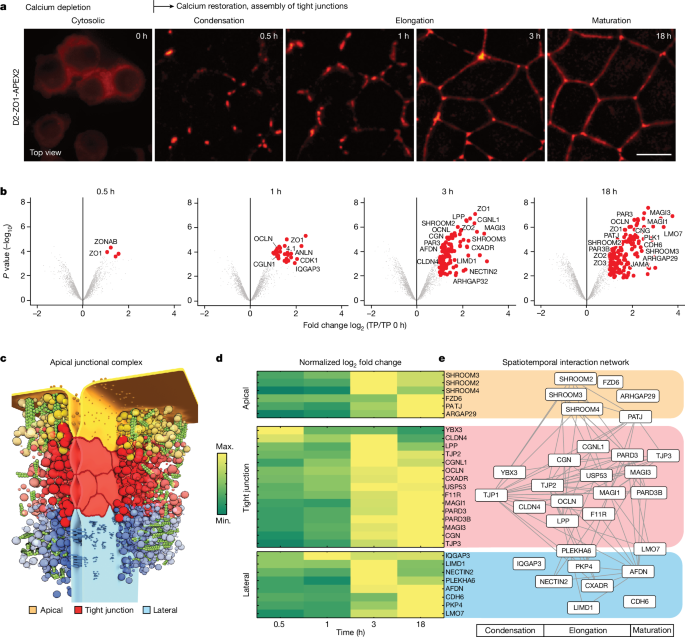
The calcium depletion experiments were performed on a confluent monolayer of MDCK-II with different cell lines. Cells were grown for 18âh in calcium-free media until tight junctions were disrupted. For proteomics experiments, medium containing calcium was added to the cells at 37â°C, 5% CO2, and each sample was prepared at 0âh, 0.5âh, 1âh, 3âh, and 18âh afterwards. For live imaging experiments, medium containing calcium was added to the cells directly on the microscope chamber at 37â°C, 5% CO2, and tight-junction formation was imaged every 1âmin for 3âh or every 30âmin for 18âh.
Transepithelial resistance
MDCK-II cells were seeded into a Corning transwell plate with membrane inserts (12âmm, 0.4âmm pore), and transepithelial resistance was measured at various time points from 1âh after seeding until 6 days. Measurements were acquired using a two-electrode resistance system from Millipore (Millicell ERS-2) inserted between the apical and basal parts of the chamber. The electric resistance was multiplied by the growth area of the transwell filters (1.12âcm2), and the resistance of the medium without cells was subtracted to give a final result in ΩâÃâcm2.
Quantification of ZO-1 belt length and cell perimeter coverage
To determine the coverage of the cell perimeter by ZO-1 (%) or the ZO-1 belt length per cell (µm per cell), we performed the following steps. We segmented the cells in the monolayer with CellPose53 using the bright-field channel as input. The outline of the CellPose segmentation was used as the cell perimeter length. We segmented the mN-ZO-1 channel through local intensity thresholding and skeletonized the segmented image to obtain the length of the ZO-1 belt using FIJI. The percentage of the cell perimeter covered by ZO-1 was calculated by the following formula: perimeter coverageâ=â(TJ length/cell periphery)âÃâ100%. The length of the ZO-1 belt per cell, LZO-1 (in μm per cell), was calculated using MATLAB as follows: LZO-1â=âsum(skeletonized ZO-1 image)/cell number.
Determination of tight-junction protein recruitment kinetics
To determine the t1/2 arrival kinetics of the mS-tagged tight-junction proteins from the two-colour time series, we segmented the condensed mN-ZO-1 signal and quantified the mScarlet intensity in the segmented ZO-1 condensates and the cytoplasm over time. Cell segmentation was done using CellPose (https://github.com/mouseland/cellpose), and ZO-1 condensate segmentation used the plugins Subtract Background and Make Binary in FIJI. To directly correct for photobleaching artefacts, we calculated the ratio between the junctional and the cytoplasmic mScarlet signal for each time point using custom MATLAB code52. Assuming that bleaching is spatially homogenous, this ratio is independent of bleaching and directly reports the enrichment of the protein in the condensed ZO-1 (tight junction). Kinetic data were fitted using a HiII slope model in Prism with a nonlinear fit variable slope (four parameters) to calculate the half-maximal effective concentration of each protein. To calculate the t1/2 values for client protein arrival, we fitted the kinetic data to a Hill slope model and determined the difference in arrival time between ZO-1 and the client protein in living cells.
Determination of ZO-1 condensate extension and eccentricity
ZO-1 condensate eccentricity was quantified on segmented ZO-1 images using the function regionprops to measure the eccentricity of segmented condensates over time in MATLAB. The eccentricity is the ratio of the distance between the focus of the ellipse and its major axis length; it takes a value between 0 and 1. (Here, 0 and 1 are degenerate cases; an ellipse whose eccentricity is 0 is a circle, whereas an ellipse whose eccentricity is 1 is a line segment.)
Extension and intensity rates
To determine the extension of the condensates over time, we used the plugin JFilament in FIJI54. The analysis directly provided the length of the condensates over time. To quantify the amount of ZO-1 material in the condensate over time, we used the segmentation of the JFilament tracks to measure the sum intensity of the condensate per time point in MATLAB. As the JFilament tracks were one-dimensional, we extended the width of the segmentation to fit the width of the condensate.
Fluorescence recovery after photobleaching
FRAP experiments in cells were carried out with the following settings on a confocal infinty-line STED microscope (Abberior Instruments). The region of interest was bleached using a 405ânm diode at 1.5âmW at the back focal plane of the objective, with 100âms pixel dwell time. Prebleaching and postbleaching images were acquired using a 490ânm laser at 5âμW. Fluorescence recovery of mNeon was monitored for 1â20âmin with a time resolution of 11âs. Cell movements during the recovery were corrected by registration of all frames to the first frame using the plugin StackReg in FIJI.
Client partitioning assays in HEK293
HEK293 cells were transfected with ZO-1âGFP, and clients were tagged with mCherry. We measured the fluorescence intensity of the client inside and outside the condensate of ZO-1, and the background outside the cell was subtracted from those values.
Immunoblotting and in-gel fluorescence
Aliquots of approximately 5âμg of input, elution and beads in lysate buffer (10% glycerol, 2% sodium dodecyl sulfate (SDS), 1âmM dithiothreitol (DTT), 1x Protease Inhibitor Cocktail, 50âmM Tris-HCl, pH 8) were subjected to SDS polyacrylamide gel electrophoresis (4â20% Tris-Glycine Novex gels) at 90âmV for 3âh. An iBlot2 gel transfer system (Thermo Fisher, IB21001) was used to transfer proteins on to a nitrocellulose membrane (10âmin, 20âV). An iBind Flex system (Thermo Fisher, SLF2000) was used to detect protein levels by immunoblotting, with the following antibodies: IRDye-800CW streptavidin (1:4000, LI-COR, 92632230). Membranes were scanned with an LI-COR Odyssey system at 700ânm and 800ânm. In-gel fluorescence of the tagged proteins (Dendra2) was done in a Typhoon FLA 7000 scan system (GE). For western blot detection of PATJ, a wet system was used for transfer (260âmA for 2âh), and classical HRP detection of proteins was done using superSignal West Pico Plus chemiluminescent substrate (Thermo Fisher, catalogue no. 34580) on GE hyperfilms (Cytiva, 28-9068-50). Primary antibody incubation was performed overnight at 4â°C in 5% milk and 0.1% Tween, with PATJ-L27 (rabbit polyclonal, gifted by Le Bivicâs lab) 1:200, PATJ-PDZ4 (rabbit polyclonal, LSBio, LC-C410011), and 1:500 β-actin (rabbit polyclonal, Abcam, ab8227). The following secondary antibodies were used at 1:5000 dilution: goat anti-rabbit IgG-HRP (H+L) (Cell Signaling, catalogue no. 7074), and goat anti-mouse IgG-HRP (H+L) (Cell Signaling, catalogue no. 7076) (Supplementary Fig. 1).
Immunofluorescence
Fixation was performed in the same way for both two-dimensional monolayers and three-dimensional cysts. Cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10âmin at room temperature, followed by quenching in 300âmM glycine, and permeabilized with 0.5% Triton X-100 in PBS for 10âmin. Cells were blocked with 2% bovine serum albumin and 0.1% Triton X-100 in PBS for 1âh at room temperature. Staining of all primary and secondary antibodies involved incubation at room temperature for 2âh or 30âmin with a dilution of 1:50 or 1:200, respectively, in blocking buffer. Staining for neutravidin-647 (neutravidin, A-2666 and Alexa Fluor 647 succinimidyl ester A-20006, Invitrogen) was performed at 1:1000 dilution for 1âh at room temperature in blocking buffer. Primary antibodies were PATJ-L27 rabbit polyclonal (produced in-house), PATJ-PDZ4 rabbit polyclonal (LSBio, LC-C410011), PALS1 mouse monoclonal (Santa Cruz, sc-365411), ZO-1 mouse monoclonal IgG1 (Invitrogen, 33-9100), occludin rabbit polyclonal (Life Technologies, 71-1500), Lin7 rabbit polyclonal (Thermo Fisher, 51-5600), E-cadherin rabbit IgG, (Cell Signalling, 3195S). The secondary antibodies were goat anti-mouse (Abberior, star-red 2-0002-011-2) and goat anti-rabbit star-orange (Abberior, storange-1102)
APEX2 labelling during tight-junction formation
Cells were plated in a T75 flask after reaching confluency, and the full medium was substituted with medium without calcium for 18âh to enable the cells to reach a rounded, non-polarized state. Afterwards, the medium was replaced with full medium again, and APEX2 labelling was performed at various time points by adding 1âmM biotin phenol to the cells for 30âmin before the addition of 1âmM H2O2 for 1âmin. Immediately after, the reaction was quenched on ice by washing three times with 1à PBS supplemented with 10âmM sodium ascorbate (Sigma A4034), 10âmM sodium azide, and 5âmM Trolox ((+/â)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid, Sigma 238813). To validate biotinylation through fluorescence microscopy, cells were immediately fixed after this step. For proteomics and western blot analyses, cells were lysed by scraping them off the growth surface into ice-cold lysis buffer (2% SDS, 10% glycerol, 1âmM DTT, 50âmM Tris-HCl, pH 8, 1à Protease Inhibitor Cocktail Set III EDTA-Free (EMD Millipore, catalogue no. 539134), supplemented with 10âmM sodium ascorbate, 10âmM sodium azide and 5âmM Trolox. The lysate in was collected in a low-protein-binding reaction tube and 5âμl of benzonase was added, followed by incubation in a shaker for 15âmin at 37â°C, 700ârpm. Finally, 1âmM EDTA and 1âmM EGTA were added, and the sample was spun down using a table centrifuge to remove debris. After lysis, the protein concentration was checked using a Pierce 660-nm (Thermo Fisher Scientific, catalogue no. 22660) protein assay test before pull-down to ensure the same starting protein concentration was used. Lysates were aliquoted into 1,500âμg of total protein, snap frozen and stored at â80â°C.
Streptavidin pull-down of APEX2 biotinylated proteins
All buffers used for pull-down proteomics experiments were freshly made and filtered with a 0.22âμm filter before use. Frozen lysates (1,500âg protein) were diluted 1:10 in 50âmM Tris, pH 8, and placed in an incubation buffer consisting of 0.2% SDS, 1% glycerol, 1âmM DTT, 1âmM EGTA, 1âmM EDTA in 50âmM Tris-HCl, pH 8, and 1à protease inhibitor. For affinity purification, approximately 100âl of streptavidin magnetic beads (Pierce, PI88817) were washed in incubation buffer two times before binding. Beads were added to the sample in a total volume of 2âml and incubated for 2âh at room temperature in a rotating wheel. Next, beads were pelleted down using a magnetic rack, and the supernatant was collected and kept for western blot analysis. Each sample of beads containing the bound biotinylated proteins was washed with a series of ice-cold buffers (2âml each) to remove unspecific binders. The beads were then washed twice with washing buffer (0.2% SDS, 1% glycerol, 1âmM DTT, 1âmM EGTA, 1âmM EDTA in 50âmM Tris-HCl pH 8, 1à protease inhibitor), once with 1âM KCl, once with 2âM urea in 50âmM Tris-HCl pH 8, once with 2âmM biotin 50âmM Tris-HCl pH 8, and finally three times with 50âmM Tris-HCl pH 8. Biotinylated proteins were eluted by boiling the beads in 50âμl of elution buffer (5% SDS, 10% glycerol, 20âmM DTT, in 50âmM Tris-HCl, pH 8, 1à protease inhibitor) at 95â°C for 10âmin, followed by cooling on ice and a brief spin-down. Samples were placed on a magnetic rack, and the eluate was collected in a new tube for proteomics and SP3. Magnetic beads and 10âμl of eluate were together subjected to western blotting for validation of the biotinylation experiments before mass spectrometry.
To process all time points in the same quantitative mass spectrometry analysis, we used a multiplex proteomic approach based on tandem mass tag (TMT). The TMT isobaric tagging approach enables robust quantitative proteomics by measuring all samples in one run. This enabled statistical analysis of relative protein enrichment at different time points after calcium switch across proteins detected at all time points.
Mass spectrometry and TMT labelling
Reduction of cysteine-containing proteins was performed with dithiothreitol (56â°C, 30âmin, 10âmM in 50âmM HEPES, pH 8.5). Reduced cysteines were alkylated with 2-chloroacetamide (room temperature, in the dark, 30âmin, 20âmM in 50âmM HEPES, pH 8.5). Samples were prepared using the SP3 protocol55,56, and 300âng trypsin (sequencing grade, Promega) was added per sample for overnight digestion at 37â°C. Peptides were labelled with TMT10plex Isobaric Label Reagent (Thermo Fisher) according to the manufacturerâs instructions. In short, 0.8âmg reagent was dissolved in 42âμl acetonitrile (100%), and 8âμl of stock solution was added, followed by incubation for 1âh and quenching with 5% hydroxylamine for 15âmin at room temperature. Samples were combined for the TMT10plex, and an OASIS HLB µElution Plate (Waters) was used for further sample clean-up57. Offline high-pH reverse-phase fractionation was performed using an Agilent 1200 Infinity high-performance liquid chromatography system equipped with a quaternary pump, degasser, variable-wavelength ultraviolet detector (254ânm), and Peltier-cooled autosampler and fraction collector (both set at 10â°C for all samples).
The column was a Gemini C18 column (3âμm, 110âà , 100âÃâ1.0âmm, Phenomenex) with a Gemini C18, 4âÃâ2.0âmm SecurityGuard (Phenomenex) cartridge as a guard column. The solvent system consisted of 20âmM ammonium formate (pH 10.0) (phase A) and 100% acetonitrile as the mobile phase (B). The separation was accomplished at a mobile phase flow rate of 0.1âmlâminâ1 using the following linear gradient: 100% A for 2âmin, from 100% A to 35% B in 59âmin, to 85% B in a further 1âmin, and held at 85% B for 15âmin, before returning to 100% A and re-equilibration for 13âmin. Thirty-two fractions were collected during liquid chromatography separation and subsequently pooled into six fractions. The first and the two last fractions of the 32 were discarded and not used at all. Pooled fractions were dried under vacuum centrifugation and reconstituted in 15âμl 1% formic acid, 4% acetonitrile, for liquid chromatography coupled with tandem mass spectrometry analysis.
Mass spectrometry data acquisition
Peptides were separated using an UltiMate 3000 RSLC nano liquid chromatography system (Dionex) fitted with a trapping cartridge (µ-Precolumn C18 PepMap 100, 5âµm, 300âµm i.d.âÃâ5âmm, 100âà ) and an analytical column (nanoEase M/Z HSS T3 column 75âµmâÃâ250âmm C18, 1.8âµm, 100âà , Waters). Trapping was carried out with a constant flow of solvent A (3% dimethyl sulfoxide, 0.1% formic acid in water) at 30âµlâminâ1 on to the trapping column for 6âmin. Subsequently, peptides were eluted through the analytical column with a constant flow of 0.3âµlâminâ1 with an percentage of solvent B (3% dimethyl sulfoxide, 0.1% formic acid in acetonitrile) increasing from 2% to 8% in 4âmin, from 8% to 28% in 104âmin, from 28% to 40% for a further 4âmin and finally from 40% to 80% for 4âmin before returning to the equilibration condition of 2%. The outlet of the analytical column was coupled directly to an Orbitrap Fusion Lumos Tribid mass spectrometer (Thermo Fisher) using the proxeon nanoflow source in positive ion mode.
The peptides were introduced into the Fusion Lumos through a Pico-Tip Emitter 360âµm ODâÃâ20âµm ID 10âµm tip (New Objective) with an applied spray voltage of 2.2âkV. The capillary temperature was set to 275â°C. A full mass scan was acquired with a mass range of 375â1500âm/z in profile mode in the Orbitrap with a resolution of 120,000. The filling time was set to a maximum of 50âms with a limitation of 4âÃâ105âions. Data-dependent acquisition was performed with the resolution of the Orbitrap set to 30,000, a fill time of 94âms and a limitation of 1âÃâ105âions. A normalized collision energy of 36 was applied. MS2 data were acquired in profile mode.
Mass spectrometry analysis
IsobarQuant and Mascot (v.2.2.07) were used to process the acquired data. The data were then searched against the UniProt Canis lupus proteome database (UP000805418), which contains common contaminants and reversed sequences. The following modifications were included in the search parameters: carbamidomethyl (C) and TMT10 (K) (fixed modifications); and acetyl (N-term), oxidation (M), and TMT10 (N-term) (variable modifications). A mass error tolerance of 10âppm and 0.02âDa was set for the full scan (MS1) and the MS/MS spectra, respectively. A maximum of two missed cleavages was allowed, and the minimum peptide length was set to seven amino acids. At least two unique peptides were required for protein identification. The false discovery rate (FDR) at the peptide and protein level was set to 0.01. The R programming language (ISBN 3-900051-07-0) was used to analyse the raw output data of IsobarQuant. Potential batch effects were removed using the limma package. Variance stabilization normalization was applied to the raw data using the vsn package. Individual normalization coefficients were estimated for different time points compared with the non-calcium condition (t0). Normalized data were tested for differential expression using the limma package. The replicate factor was included in the linear model. For comparisons of different time points versus the t0 condition, proteins with fold change greater than 2 were considered to be potential hits, and an FDR threshold of 0.05 was used to filter out noisy data. In addition, we excluded false positive hits due to non-junctional interactions (ribosomes, nucleus, mitochondria, endoplasmic reticulum (Supplementary Table 2).
In experiments comparing different time points, proteins were first tested for their enrichment compared with a ât0 control. R package fdrtool35 was used to calculate FDRs using the t values from the limma output. Proteins with an FDR less than 5% and a consistent fold change of at least 10% in each replicate were defined as hits. The ggplot2 R package was used to generate graphics. Proteins matching a false positive list of non-biotinylated proteins were removed (Supplementary Table 3). RStudio code was adapted from previously reported code (https://github.com/fstein/EcoliTPP). R packages used were limma (https://bioconductor.org/packages/limma), MSnbase (https://bioconductor.org/packages/MSnbase), tidyverse (https://tidyverse.tidyverse.org), biobroom (https://bioconductor.org/packages/biobroom), ggrepel (https://cran.r-project.org/web/packages/ggrepel/vignettes/ggrepel.html) and ClusterProfiler (https://bioconductor.org/packages/clusterProfiler/). The interactome was created in Cytoscape (v.3.9.0) using the STRING database (v.11.5). Cell localization gene ontology annotations were from http://geneontology.org. The mass spectrometry proteomics data have been deposited at the ProteomeXchange Consortium through the PRIDE58 partner repository with dataset identifier PXD052221.
Quantification and statistical analysis
Images were analysed with FIJI (https://fiji.sc/) and MATLAB (MathWorks). All data are expressed as the meanâ±âs.d., meanâ±âs.e.m. or meanâ±â95% confidence interval, as stated in the figure legends and results. Values of n and what n represents (for instance, number of images, condensates or experimental replicates) are stated in figure legends and results. Two-tailed Studentâs t-test or one-way ANOVA was used for normally distributed data. Statistical analyses used KruskalâWallis test with post hoc Dunnâs multiple-comparison test.
Numerical calculations for the thermodynamic model
Numerical calculations were done using programming language Python v.3.8.10; all code was run using IPython v.7.3.10. This software comes preinstalled in most of the Linux distributions. OS: Ubuntu 20.04.6 LTS, 64-bit, GNOME v.3.36.8. All code and a minimal dataset are available at Zenodo (https://zenodo.org/doi/10.5281/zenodo.11174400)52.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.